Click Here if you listened. We’re trying to gauge interest so only one question is required; however, there is a spot for feedback!
Read along below!
Found in Translation
Mite Drop!
By: Jay Evans, USDA Beltsville Bee Lab
Varroa mites remain the primary source of honey bee colony losses for beekeepers managing from one to 10,000 colonies. Scientists like us and ardent beekeepers are always on the hunt for new ways to reduce varroa damage to bees and their colonies. One intriguing strategy is to make mites simply fall off their adult bee hosts. Short of changing the electric charge of host or parasite, this repellency can come from 1) making hosts less grippy, 2) somehow clogging the incredibly strong tarsi (feet with ‘toes’ and a spongy, oily, arolia) of mites or 3) affecting mite behavior by making them less likely to find safe spots and hang on to their bees for dear life. Dislodged mites are far more vulnerable to hygienic worker bees and might also simply keep falling down to a hostless, hungry and hopefully, short life. This is probably a central reason that female varroa mites spend very little time wandering the combs of beehives unless they are moments away from entering the brood cell of a developing bee. While on adult bees, mites have much incentive to stay right there, whatever their host is doing to drop them.
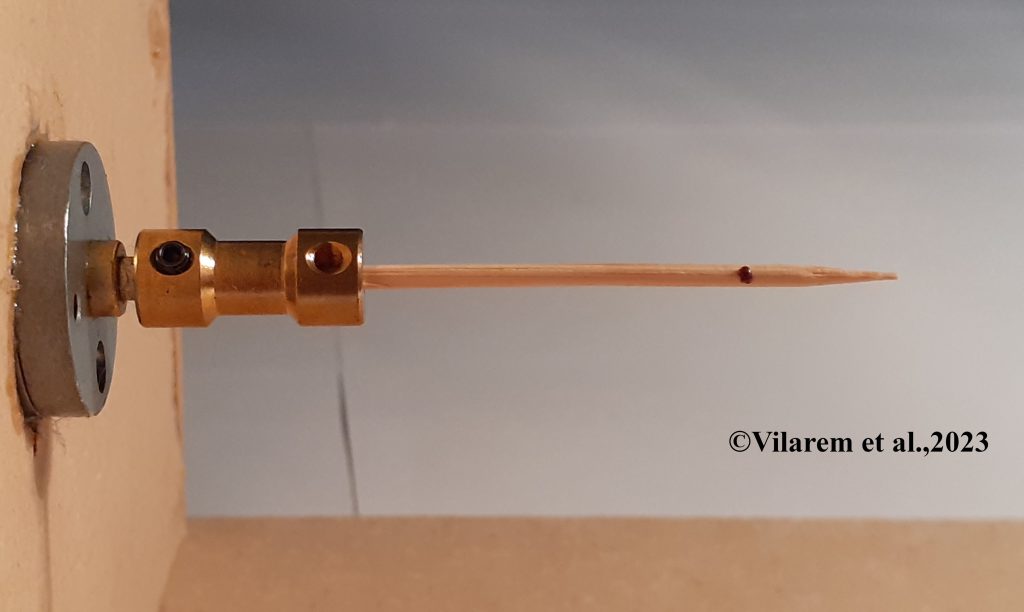 How do mites adhere to their bees so strongly? When mites are actively feeding on bees they are extremely hard to dislodge, since they are partly under the hardened plates of the bee itself and are gripping with a combination of ‘teeth’ and tarsi. Even while taking a break from feeding, mites know to find safe spots on the bee to attach, favoring locations on the abdomen or thorax that are both hairy and away from swinging legs and biting bee mandibles. How can one make them quit their bees given so many hiding places?
How do mites adhere to their bees so strongly? When mites are actively feeding on bees they are extremely hard to dislodge, since they are partly under the hardened plates of the bee itself and are gripping with a combination of ‘teeth’ and tarsi. Even while taking a break from feeding, mites know to find safe spots on the bee to attach, favoring locations on the abdomen or thorax that are both hairy and away from swinging legs and biting bee mandibles. How can one make them quit their bees given so many hiding places?
Caroline Vilarem and colleagues in France recently described an ambitious attempt to document the abilities of mites to hang onto surfaces when exposed to organic acids (Vilarem, C.; Piou, V.; Blanchard, S.; Vogelweith, F.; Vétillard, A. Lose Your Grip: Challenging Varroa destructor Host Attachment with Tartaric, Lactic, Formic, and Citric Acids, Appl. Sci. 2023, 13, 9085. https://doi.org/10.3390/app13169085). These scientists deployed one of the coolest low-tech tools to measure how well mites grip onto a surface. While their ‘Rotavar’ sounds both complex and expensive, it is actually a ‘motor-driven rotating toothpick’. Yes, you can do this at home, with a slow (three or so revolutions per minute) motor and a supply of toothpicks. The authors add to that an extremely careful experimental design and complex statistics to show the different abilities of mites to hang onto sticks and bees coated with acetic, citric, lactic, formic and tartaric acids. The results hint at new modes and new candidates for mite control, with the usual caveat that converting a controlled lab assay to field colonies will be challenging.
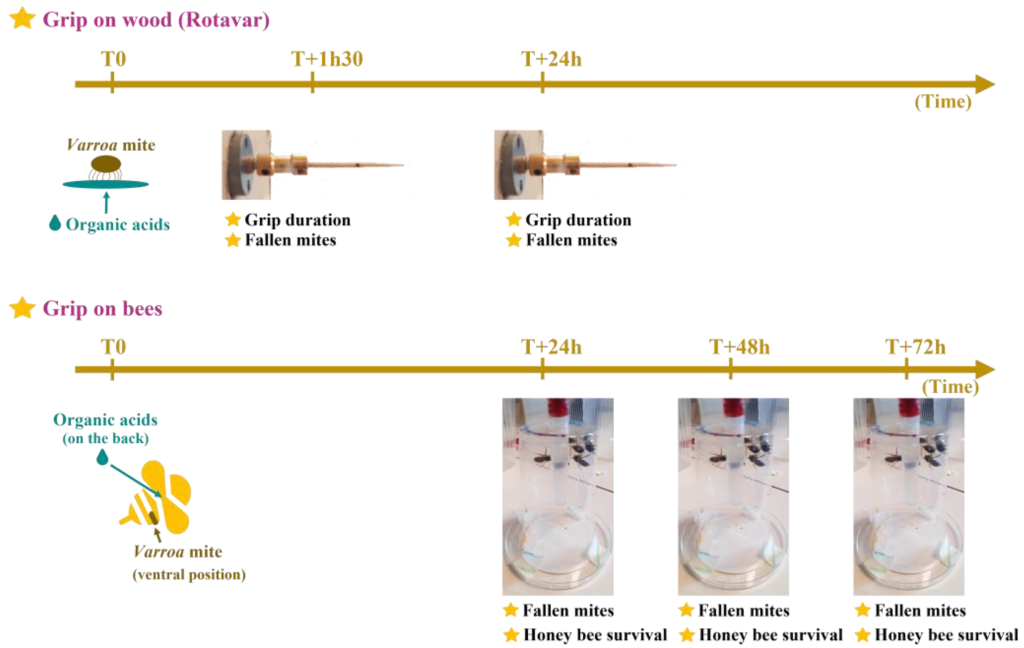
Schematic diagram of the experimental design and measured parameters. Grip on wood (Rotavar): This method relies on direct contact between Varroa’s arolia and the organic acids. The Rotavar set-up is a motor-driven rotating toothpick used to assess V. destructor’s grip. Grip on bees: the host attachment experiment applies acids to the backs of honey bees to remove mites. T0 represents the administration time for treatments; T + 1 h 30, 24 h, 48 h, or 72 h stand for the time post administration used to make measurements. Figure from https://doi.org/10.3390/app13169085
Some highlights: First, acidity itself does not seem to be the solution. Most notably, even high doses of acetic acid had little impact on the abilities of mites to grab toothpicks and this candidate was quickly discarded. So, what can we glean from the differences between the tested acids? Tartaric acid worked great at dislodging mites from spinning toothpicks but was surprisingly poor at dislodging mites from bees. Prior work suggests that the mode of action for tartaric acid is, at least in part, toxicity towards mites. It is possible that the levels of tartaric acid needed to coat bees with a toxic dose are higher than they are on a relatively smooth and barren toothpick. Toothpicks also attract watery compounds (hydrophilic) while bees are coated with oils and are hence more water-repellent (hydrophobic). Maybe the availability of tartaric acid on toothpicks is higher than it would be on oilier bee bodies. Formic acid also worked much better on the wood surface than on bees, an intriguing insight for a well-used and effective mite control. Formic acid is also known to be directly toxic to mites and their cells, and the authors make clear that both direct toxicity and grippiness are clear and perhaps synergistic targets for mite control. The widely used miticide oxalic acid also wins by being directly toxic to mites at levels that are relatively safe for bees, demonstrating that there are many possible ways to turn organic acids into effective treatments.
Lactic acid came out as the best candidate in the study group for divorcing mites from their bees. This acid worked well at dislodging mites from both toothpicks and bees. Lactic acid does not appear to be highly toxic to mites and instead seems to act by changing the mechanics of hanging on. This is a nice lead for exploring acids with similar qualities for their abilities to both grease the ‘Rotavar’ and make bees a more slippery host. In another intriguing result from this nice study, mites that simply walked across paper holding lactic acid were then less good in future grip tests. What is it about lactic acid that burns, cleans or otherwise insults the complex and surprisingly ‘soft’ tarsi of mites?
If this topic has gripped you, consider reading up on the field thanks to a recent open-access paper on stickiness by graduate student Luc van den Boogaart and colleagues in the Netherlands (van den Boogaart, L.M.; Langowski, J.K.A.; Amador, G.J. Studying Stickiness: Methods, Trade-Offs, and Perspectives in Measuring Reversible Biological Adhesion and Friction. Biomimetics 2022, 7, 134; https://www.mdpi.com/2313-7673/7/3/134). For those of us who have stored ‘Freshman Physics’ in a remote hard drive, they give a clear review of how these forces work across organisms; in their words ‘from ticks to tree frogs’. Maybe their figures and insights will inspire a beekeeper or scientist to dream up a safe, effective route to dislodge mites from bees and prevent them from climbing back on. Pulling in people with a knowledge of physics, or just really good imaginations and the ability to build and deploy Rotavars (imagine how entertaining those can be, a la squirrel spinners… https://www.youtube.com/shorts/nBKb_z4_tGY), can only help in the hunt for new mite controls and healthier bees.
]]>By: Nina Bagley
 Miss Lillian Love was born into a Quaker family in Marion, Indiana on October 24, 1880. Her family was from Decatur, Indiana. Her father, Granville Love, was born in Indiana. In 1860, he married Nancy J. Gillibrand. Her family came from England and settled in the vicinity of Indianapolis. The two were married on August 11, 1868, in Morgan County, Indiana. Granville was a farmer and ran a huckster wagon, which proved a good business. Mrs. Nancy Love had nine children from 1869 to 1892. She died on January 31, 1936, at eighty-six, in Decatur, Indiana. Lillian’s father, Granville Love, died May 7, 1925, in Guilford, Indiana. All the children would be trained in English and piano at Central Normal College in Danville, Indiana.
Miss Lillian Love was born into a Quaker family in Marion, Indiana on October 24, 1880. Her family was from Decatur, Indiana. Her father, Granville Love, was born in Indiana. In 1860, he married Nancy J. Gillibrand. Her family came from England and settled in the vicinity of Indianapolis. The two were married on August 11, 1868, in Morgan County, Indiana. Granville was a farmer and ran a huckster wagon, which proved a good business. Mrs. Nancy Love had nine children from 1869 to 1892. She died on January 31, 1936, at eighty-six, in Decatur, Indiana. Lillian’s father, Granville Love, died May 7, 1925, in Guilford, Indiana. All the children would be trained in English and piano at Central Normal College in Danville, Indiana.


Lillian’s parents, Granville and Nancy Love.
Lillian had two years of college, became a teacher, and taught in Indiana and Florida. Women still couldn’t find jobs other than teaching. Lillian and her youngest sister Flossie were involved in women’s rights and equal opportunity for women; they supported women’s rights to vote.
In 1904, Lillian moved to Tacoma, Washington, where she taught for several years. Finding her husband in 1907, she married Jay Levant Hill, who was twenty-five years older than her. Lillian’s husband, Jay, was an inventor who owned his own lumber business. Lillian said that her husband’s “brain was mechanically bent.”

Miss Lillian Love, taken in Washington State prior to her marriage to J. Levant Hill.
Their home would be Mount Shadow Ranch, a two-hundred-acre farm with a charming yellow California-style bungalow, two and a half miles from Elbe, Washington. Surrounding the farm were the bee’s favorite purple hills of fireweed.
Fireweed is a plant that enjoys cool and moist climates and thrives in Pacific Northwest forestlands. It is also considered one of the most prolific plants for honey production, with its nectar having a high sugar concentration. It has a “lightly spicy” or “buttery” flavor.
If you shut your eyes and listen, you can hear the train whistle in the distance as it stops at Park Junction Station in the middle of Mount Shadow Ranch.


1926 – Lillian Hill at Mt. Rainier
Apiary. Image featured in American Bee Journal
“We have some thirty acres under cultivation,” said Mrs. Hill; The rest of the farm is logged-off land which we use to pasture our herd of fifty cattle.”
—American Bee Journal, April 1926.
One day in 1913, an old man came peddling bees. “You have a wonderful place here for bees,” he said, convincing Mrs. Hill to invest in six swarms. Before the older man came along, she had never seen a swarm of bees before. Her six swarms increased and produced so much honey that in 1914 Lillian Hill invested in twenty more swarms, giving her forty hives. That year she harvested 6,500 pounds of pure honey. Lillian had an entrepreneurial spirit and determination to succeed in a male-dominated industry.

Lillian’s marriage to J. Hill.
I don’t know how she accomplished so much. Mrs. Lillian Hill kept a tidy home, raised beef cattle, Duroc-Jersey hogs, geese and ducks, and grew vegetables in the garden. But it would be the bees she loved the most!
Lillian was part owner of the Ranch and owner of the Mount Shadow Apiary. She had a gentle personality. She was independent and had a fire in her eyes that you could see demanded respect.
Being a novice beekeeper found her unprepared. In 1915, she encountered her first obstacle, European Foulbrood that would bring havoc to her beeyard. Words that no beekeeper wanted to hear or experience, the only cure, the dreadful burning of the hives. After that horrible experience with “American Foulbrood,” she only kept a dozen colonies of bees providing honey for her family and neighbors. (American Bee Journal, April 1926.)
“I never camp,” confessed Lillian Hill. “On either the trail of my successes or my failures. I go right on.” That’s her philosophy in a nut-shell.
Although childless, she cared for the children from reform schools, orphan asylums or neighboring farms; she taught the boys and girls everything about beekeeping so they could pay their way through school. She believed that the best and safest way to help any human being is to help him help himself. Particularly, those who needed guidance and education.
In the 1900s, the U.S. was a diverse nation, and its children lived in various circumstances. For years, she had been the leader of the Boys’ and Girls’ Bee Club of Elbe. One of her boys won nearly $80.00 with his exhibits of bees and honey at the Western Washington Fair.
Lillian increased her hives to twenty-six to help one of her boys and it didn’t stop there!
In 1924, to help one of her girls through school, she invested in thirty more hives and loaned them to the girl. The girl lived next door to an abandoned schoolhouse on an acre of ground, which got Mrs. Hill thinking, “I could rent the schoolhouse and land from the school board.”

1921 – Freddie May with his siblings before they were placed in the Washington Children’s Home.
One day in 1924, a young man showed up at the Ranch. His name was Freddie May, and he was born in 1912 in Denver, Colorado. When he was eight years old, he lived in Wenatchee, Washington. His father abandoned the family, and their mother could not care for six children. The children were placed in the Washington Children’s Home in Wenatchee, Washington, in 1921.

Mount Rainier Apiary. Freddie May and Mrs. Lillian Hill.
Freddie somehow got his hands on a newspaper. He came across the ad for a permanent position in beekeeping work. Freddie wanted to learn about the beekeeping business under the leadership of Mrs. Lillian Hill, so he rode on “a bicycle” from Wenatchee, Washington to Elbe, Washington, a hundred and ninety five miles! He was energetic and full of fire and wanted to learn beekeeping.
Lillian took a liking to Freddie and wanted to help him make money to pay his way through school, so she furnished Freddie with plenty of bees on a commission basis of fifty-fifty. In four months, the Colorado cyclist made five hundred dollars for himself.
Freddie would consider Mrs. Lillian Hill his mother and next of kin. Lillian and her husband would become Freddie’s foster parents giving him a home with security. He would attend Eatonville High School and work on the Ranch. He would continue beekeeping and eventually marry and have a family.
During the season of 1925, Mrs. Hill was able to establish the Colorado youth in the schoolhouse helping young boys and girls in need teaching them beekeeping.
In 1926, Lillian Hill had over one hundred and fifty hives of Italian bees, eighty-five at the schoolhouse and sixty-five at home. She produced at least 10,000 sections of comb honey. Mrs. Hill would advertise in the newspapers to get workers “Wanted – an experienced farmer for a permanent position.”
Most of the marketing she did herself in her Buick car. She supplied the best stores in Tacoma and Seattle. “I don’t have to hunt for a market,” declared this energetic woman. In one year, Mrs. Hill raised sixty queens. That was the part of her business that she enjoyed most of all. Mrs. Hill was the president of the Pierce County Beekeepers’ Association for two years.

Mount Rainier
Both triumphs and disasters have knocked often at Lillian Hill’s door on the Mount Shadow Ranch, but neither one ever fazed her. This woman had grit and plenty of it!
Around 1927, Freddie would accidentally run over Lillian’s foot crushing it while she was teaching him how to drive the tractor. An unfortunate outcome was that the doctors had to amputate her leg due to blood poisoning. Lillian had a prosthetic leg from the knee down, but that didn’t stop her. She took it in stride and persevered. In 1929, unfortunately, her husband died. He was the youngest of five and the last of his siblings. He was seventy-one years old. I will say some lives have more trial or tribulations than others, to be sure, but no life is without events that test and challenge us.
In the 1930 census, Lillian is listed as a widow forty-nine years old, with fifty men aged eighteen to sixty-six listed as boarders at the Mount Shadow Ranch and working for Lillian Hill. That’s a lot of men to manage. You would have to have grit and be firm! Among the fifty men working on the farm was Freddie May, the youngest, who was eighteen. His occupation was a Logger.
Not being able to care for the Ranch and losing her husband, not to mention the tractor accident, left her feeling like it would be time to sell the Ranch. Lillian Hill would place the Ranch up for sale.
Advertised in the Tacoma Daily Ledger Sunday, June 23, 1929. “Mountain Shadow Ranch. It is one of the best-stocked Dairy Farms in western Washington, with running water in every field and excellent soil. Forty acres cleared; 120 acres fenced for hogs and cattle; stocked and making money; good seven-room house with school buses to Elbe and Eatonville high school. The farm is a must-see to appreciate it. We will consider small trade—a price of $15,000. Write to Lillian L. Hill for an appointment.”

Family photo of Albert Cook, first wife Nora and their children.

Lillian’s family Bible.
A lot happened in 1930. The Ranch sold, and Lillian Hill married Albert Cook, a widower who worked in the lumber industry. His wife Nora passed away in March of 1929 at the age of fifty-one; they had six children together. Lillian didn’t mind an extended family. She was raising her niece Esther who she adopted at a young age and her foster son Freddie May. After all, Lillian loved children and teaching. Her first husband was in the lumber business so she probably knew Albert Cook.
Albert would marry Lillian in 1930, build apartment buildings and retire from the lumber industry. The two would live in Tacoma, Washington. Lillian’s beekeeping days came to an end, her new occupation would be owner and landlord of her apartment buildings.
Lillian had a very loving relationship for nineteen years with her husband Albert. In May of 1949, Albert passed away at the age of seventy-three. He was buried beside his first wife, Nora, in Tacoma, Pierce, Washington.
The income from the apartments and other investments would give Lillian a comfortable life for the next sixteen years. She lived to be eighty-eight and passed away August 19, 1969 in Tacoma, Pierce, Washington Lillian was a Sixth Avenue Baptist church member. She was buried next to her first husband, Jay Levant Hill.
Freddie May lived to be eighty-three years old. Freddie kept his surname May. He went by Fred (Cook) May, Sr. “Commander” as best by everyone who loved him.

Lillian in her sister Flossie’s backyard. Her dress is purple and black print. She and her sister Flossie always had a matching rhinestone necklace.
Lillian’s youngest sister Flossie, who she remained close with, lived in California. Flossie had a granddaughter Karla who enjoyed her aunt Lillian’s visits. She remembers sitting on her grandmother’s hunter green “davenport” with her aunt Lillian. Her grandma Flossie would sit in her desk chair across the room and the two sisters would talk for hours.
Lillian’s great-niece Karla also remembers how “intriguing” her aunt was. Lillian had blue eyes, was fair-haired and had rosy cheeks. She wore her hair in a braid reaching her waist until one day; she cut it off, curled it up, and put it in a small box for keeping. Karla remembers her Aunt Lillian as sweet but at the same time, tough and gutsy!
Marcus Aurelius was a stoic philosopher. His quote reminded me of Mrs. Lillian Love, her struggles as a woman in the 1900’s and how she put others before her, passing her knowledge about beekeeping on to so many young boys and girls in need. I would like to thank Lillian’s great-niece Karla Babcock for sharing her memories of her Aunt Lillian and grandmother Flossie.
“A life of sacrifice and putting the well being collective first, just like the bees.”
—Marcus Aurelius
Ohioqueenbee
Nina M. Bagley
Columbus, Ohio.
 Nestmate Recognition
Nestmate RecognitionBy: Clarence Collison
Pheromones are involved in intraspecific chemical communication; however, the glands associated with compounds used in nestmate recognition in honey bees remain elusive. This search is difficult since nestmate cues can arise from both within the colony, and from the environment (Kalmus and Ribbands, 1952). For example, Downs and Ratnieks (1999) found no evidence that honey bee guards used heritable cues; instead, guards appear to rely exclusively on environmental cues to distinguish nestmates from non-nestmates. However, nestmate cues can also be produced by the individual, and thus must be under genetic control (Breed, 1983; Page Jr. et al., 1991). A further factor is that the wax used to build comb in the colony is both produced and manipulated by the bees, which means it may be a medium into which recognition cues are transferred (Breed et al., 1998). Therefore, Breed et al. (1998) stated that no single factor is responsible for nestmate recognition in honey bees; rather, all three factors (genetically determined cuticular signatures, exposure to comb wax, and environmental cues e.g. floral cues) seem to work together (Martin et al., 2018).
Comb wax in honey bee colonies serves as a source and medium for transmission of recognition cues. Worker honey bees learn the identity of their primary nesting material, the wax comb, within an hour of emergence. In an olfactometer, bees discriminate between combs on the basis of odor; they prefer the odors of previously learned combs. Representatives of three of the most common compound classes in bee’s wax were surveyed for effects on nestmate discrimination behavior. Hexadecane, octadecane, tetracosanoic acid and methyl docosanoate make worker honey bees less acceptable to their untreated sisters. Other similar compounds did not have this effect. These findings support the hypothesis that nestmate recognition in honey bees is mediated by many different compounds, including some related to those found in comb wax (Breed and Stiller, 1992).
Breed et al. (1998) investigated how kin recognition cues develop and cue differentiation between honey bee colonies. Exposure to the wax comb in colonies is a critical component of the development of kin recognition cues. In this study, they determined how the cues develop under natural conditions (in swarms), whether the genetic source and age of the wax affect cue ontogeny, and whether exposure to wax, as in normal development, affects preferential feeding among bees within social groups. Cue development in swarms coincided with wax production, rather than with the presence of brood or the emergence of new workers; this finding supported previous observations concerning the importance of wax in cue ontogeny. Effective cue development required a match between the genetic source of the workers attempting to enter the hive, the wax to which they were exposed and the guards at the hive entrance. The wax must also have been exposed to the hive environment for some time. Cues gained from wax did not mask or override cues used in preferential feeding interactions; this finding supports the contention that two recognition systems, one for nestmate recognition and the other for intra-colonial recognition, are present.
Recognition of nestmates from aliens is based on olfactory cues, and many studies have demonstrated that such cues are contained within the lipid layer covering the insect cuticle. These lipids are usually a complex mixture of tens of compounds in which aliphatic hydrocarbons are generally the major components. Dani et al. (2005) tested whether artificial changes in the cuticular profile through supplementation of naturally occurring alkanes and alkenes in honey bees affect the behavior of nestmate guards. Compounds were applied to live foragers in microgram quantities and the bees returned to their hive entrance where the behavior of the guard bees was observed. In this fashion, they compared the effect of single alkenes with that of single alkanes; the effect of mixtures of alkenes versus that of mixtures of alkanes and the whole alkane fraction separated from the cuticular lipids versus the alkene fraction. With only one exception (the comparison between n-C19 and (Z)9-C19), in all the experiments bees treated with alkenes were attacked more intensively than bees treated with alkanes. This led them to conclude that modification of the natural chemical profile with the two different classes of compounds has a different effect on acceptance and suggests that this may correspond to a differential importance in the recognition signature.
 Cuticular hydrocarbons (CHCs) function as recognition compounds in honey bees. It is not clearly understood where CHCs are stored in the honey bee. Martin et al. (2018) investigated the hydrocarbons and esters found in five major worker honey bee exocrine glands, at three different developmental stages (newly emerged, nurse and forager) using a high temperature GC analysis. They found the hypopharyngeal gland contained no hydrocarbons nor esters, and the thoracic salivary and mandibular glands only contained trace amounts of n-alkanes. However, the cephalic salivary gland (CSG) contained the greatest number and highest quantity of hydrocarbons relative to the five other glands with many of the hydrocarbons also found in the Dufour’s gland, but at much lower levels. They also discovered a series of oleic acid wax esters that lay beyond the detection of standard GC columns. As a bee’s activities changed, as it aged, the types of compounds detected in the CSG also changed. For example, newly emerged bees have predominately C19-C23n-alkanes, alkenes and methyl-branched compounds, whereas the nurses’ CSG had predominately C31:1 and C33:1 alkene isomers, which are replaced by a series of oleic acid wax esters in foragers. These changes in the CSG were mirrored by corresponding changes in the adults’ CHCs profile. The CSG is a major storage gland of CHCs. As the CSG duct opens into the buccal cavity (mouth), the hydrocarbons can be worked into the comb wax and could help explain the role of comb wax in nestmate recognition experiments.
Cuticular hydrocarbons (CHCs) function as recognition compounds in honey bees. It is not clearly understood where CHCs are stored in the honey bee. Martin et al. (2018) investigated the hydrocarbons and esters found in five major worker honey bee exocrine glands, at three different developmental stages (newly emerged, nurse and forager) using a high temperature GC analysis. They found the hypopharyngeal gland contained no hydrocarbons nor esters, and the thoracic salivary and mandibular glands only contained trace amounts of n-alkanes. However, the cephalic salivary gland (CSG) contained the greatest number and highest quantity of hydrocarbons relative to the five other glands with many of the hydrocarbons also found in the Dufour’s gland, but at much lower levels. They also discovered a series of oleic acid wax esters that lay beyond the detection of standard GC columns. As a bee’s activities changed, as it aged, the types of compounds detected in the CSG also changed. For example, newly emerged bees have predominately C19-C23n-alkanes, alkenes and methyl-branched compounds, whereas the nurses’ CSG had predominately C31:1 and C33:1 alkene isomers, which are replaced by a series of oleic acid wax esters in foragers. These changes in the CSG were mirrored by corresponding changes in the adults’ CHCs profile. The CSG is a major storage gland of CHCs. As the CSG duct opens into the buccal cavity (mouth), the hydrocarbons can be worked into the comb wax and could help explain the role of comb wax in nestmate recognition experiments.
Worker honey bees are able to discriminate between combs on the basis of genetic similarity to a learned comb. The nestmate recognition cues that they acquire from the comb also have a genetically correlated component. Cues are acquired from comb in very short exposure periods (five minutes or less) and can be transferred among bees that are in physical contact. Gas chromatographic analysis demonstrates that bees with exposure to comb have different chemical surface profiles than bees without such exposure. These results support the hypothesis that comb-derived recognition cues are highly important in honey bee nestmate recognition. These cues are at least in part derived from the wax itself, rather than from floral scents that have been absorbed by the wax (Breed et al., 1995).
Experiments indicated that the most important recognition pheromones are the fatty acids, particularly palmitic acid, palmitoleic acid, oleic acid, linoleic acid, linolenic acid and tetracosanoic acid. These fatty acids are mixed with the wax hydrocarbons from wax glands, molded into comb and then transferred onto the workers as they contact the comb. The result is a colony level signature that varies little among workers in a colony. Newly emerged workers have few external fatty acids or hydrocarbons. Oleic acid is more abundant than the other fatty acids on newly emerged bees, but the amount of oleic acid on the cuticle does not vary significantly among colonies. Newly emerged workers are accepted even though they have no signature yet; the “password” for new bees to be admitted to their colony is apparently the lack of a signal. This conclusion is corroborated by the finding that guards tend to treat sodium hydroxide-washed older bees as if they are newly emerged (Breed, 1998).
The integration of recognition cues is described as follows. Fatty acids and hydrocarbons are components of the wax comb that is produced by the bees. The relative abundances of fatty acids and hydrocarbons in wax varies among colonies, giving them unique chemical signatures. Food odors may also be absorbed by the comb, adding to its uniqueness. Newly emerged bees produce their own hydrocarbon coating, which is modified as they move around the nest by the addition of hydrocarbons and fatty acids from the comb. Of the compounds tested in the laboratory, fatty acids are the most important recognition pheromones, but other, as yet untested compounds may also contribute to the recognition odor. Hydrocarbons have generally been assumed to be the primary recognition pheromones of honey bees. However, none of the major structural hydrocarbons of honey bees (i.e., n-alkanes) yields a positive result in a recognition bioassay, nor do these compounds differ significantly in relative concentration among families of bees (Breed, 1998).
The environmental and genetic components of recognition are difficult to separate even in controlled conditions. Getz and Smith (1983) showed that the honey bee discriminates between full and half-sisters raised in the same hive, on the same brood comb in neighboring cells, thus demonstrating a significant genetic component to the recognition process.
Nestmate recognition information can come from either contact chemoreception or olfaction. Mann and Breed (1997) investigated what role airborne olfactory cues play in nestmate recognition by honey bee colony guards, and how do these signals affect guard orientation and behavior? They demonstrated that airborne cues play a significant role in guard bee recognition of nestmates and non-nestmates. Exposure of a guard bee to the scent of a non-nestmate resulted in increased locomotory rate and changes in the directional orientation of guard bees. Exposure to scent of a non-nestmate did not, however, increase the likelihood that a second non-nestmate would be attacked when placed with the guard. Observations of guard behavior at colony entrances indicate that guards discriminate nestmates from non-nestmates with high efficiency.
Floral oils are an important component of the honey bee’s olfactory environment. Bowden et al. (1998) used laboratory and field tests to determine whether floral oils affect nestmate recognition in honey bees. In the laboratory, newly emerged worker bees, that have not been exposed to comb wax, responded more aggressively to bees that had been exposed to floral oils than unexposed control bees. In the field, guard bees did not respond differently to foragers that had been exposed to floral oils. Floral oils may play a supplementary role in nestmate recognition; however, if they have any effect, it is secondary to cues acquired from comb during development.
Downs et al. (2000) investigated the effect that floral oils (anethole, citronellal, limonene and linalool) have on the probability of nestmates and non-nestmates being accepted by guard bees at nest entrances. Floral oils did not affect the probability of workers, either nestmates or non-nestmates, being accepted by guards. However, the presence of floral oils did increase the time taken for a guard to reject an introduced bee. These data show that guards are sensitive to floral oils but use other recognition cues when assessing colony affiliation.
Honey bees have the ability to distinguish among groups of larvae that are destined to become queens and preferentially rear highly related nestmate larvae over less related larvae that are not nestmates (Page and Erickson, 1984).
Colonies of honey bees from two patrilines (cordovan and dark) were established and observations were made on the behavior shown by the worker bees in rearing queen larvae within their colonies. The relationship among the bees within these colonies was either r = ¾ (super-sisters) or r = ¼ (half sisters). The worker bees showed preferential care to the queen larvae that were of their own patriline. Workers of the cordovan patriline showed a stronger preference for larvae of their own patriline than did the dark workers. Cordovan workers also showed a higher rate of visitation, indicating behavioral differences between the patrilines. These results suggest that kin selection is operating on honey bee behavior used in rearing reproduction (Noonan, 1986).
A honey bee queen is usually attacked if she is placed among the workers of a colony other than her own. This rejection occurs even if environmental sources of odor, such as food, water and genetic origin of the workers, are kept constant in laboratory conditions. The genetic similarity of queens determines how similar their recognition characteristics are; inbred sister queens were accepted in 35% of exchanges, outbred sister queens in 12% and non-sister queens in 0%. Carbon dioxide narcosis (stuper, unconsciousness) results in worker honey bees accepting non-nestmate queens. A learning curve is presented, showing the time after narcosis required by workers to learn to recognize a new queen. In contrast, workers transfer results in only a small percentage of the workers being rejected. The reason for the difference between queens and workers may be because of worker and queen recognition cues having different sources (Breed, 1981).
Boch and Morse (1974, 1979) have shown that honey bee queens can be recognized individually by swarms of bees. They found that marking a queen with shellac-based paint to give her a distinctive odor resulted in workers later exhibiting a preference for any queen marked with that paint. However, their experiments do not show whether the odors used by workers to recognize queens are produced by the queens or are environmentally acquired. In a series of studies concerned with queen introduction into colonies, Szabo (1974, 1977) also found that workers could discriminate among queens, but did not approach the issue of the source of recognition odors directly. It was also found that factors such as the age and weight of an introduced queen could affect worker choice among introduced queens. Yadava and Smith (1971) found that the mandibular gland contents of the queen were important in the release of worker aggression towards an introduced queen (Breed, 1981).
References
Boch, R. and R.A. Morse 1974. Discrimination of familiar and foreign queens by honey bee swarms. Ann. Entomol. Soc. Am. 67: 709-711.
Boch, R. and R.A. Morse 1979. Individual recognition of queens by honey bee swarms. Ann. Entomol. Soc. Am. 72: 51-53.
Bowden, R.M., S. Williamson and M.D. Breed 1998. Floral oils: their effect on nestmate recognition in the honey bee, Apis mellifera. Insectes Soc. 45: 209-214.
Breed, M.D. 1981. Individual recognition and learning of queen odors by worker honey bees. Proc. Nat. Acad. Sci. USA. 78: 2635-2637.
Breed, M.D. 1983. Nestmate recognition in honey bees. Anim. Behav. 31: 86-91.
Breed, M.D. 1998. Recognition pheromones of the honey bee. Bioscience 48: 463-470.
Breed, M.D. and T.M. Stiller 1992. Honey bee, Apis mellifera, nestmate discrimination: hydrocarbon effects and the evolutionary implications of comb choice. Anim. Behav. 43: 875-883.
Breed, M.D., M.F. Garry, A.N. Pearce, B.E. Hibbard, L.B. Biostad and R.E. Page, Jr. 1995. The role of wax comb in honey bee nestmate recognition. Anim. Behav. 50: 489-496.
Breed, M.D., E.A. Leger, A.N. Pearce, and Y.J. Wang 1998. Comb wax effects on the ontogeny of honey bee nestmate recognition. Anim. Behav. 55:13-20.
Dani, F.R., G.R. Jones, S. Corsi, R. Beard, D. Pradella and S. Turillazzi 2005. Nestmate recognition cues in the honey bee: differential importance of cuticular alkanes and alkenes. Chem. Senses 30: 477-489.
Downs, S.G. and F.L.W. Ratnieks 1999. Recognition of conspecifics by honey bee guards (Apis mellifera) uses non-heritable cues applied to the adult stage. Anim. Behav. 58: 643-648.
Downs, S.G., F.L.W. Ratnieks, S.L. Jefferies, and H.E. Rigby 2000. The role of floral oils in the nestmate recognition system of honey bees (Apis mellifera L.). Apidologie 31: 357-365.
Getz, W.M. and K.B. Smith 1983. Genetic kin recognition: honey bees discriminate between full and half sisters. Nature 302: 147-148.
Kalmus, H. and C.R. Ribbands 1952. The origin of the odours by which honey bees distinguish their companions. Proc. R. Soc. Lond. B. 140: 50-59.
Mann, C.A. and M.D. Breed 1997. Olfaction in guard honey bee responses to non-nestmates. Ann. Entomol. Soc. Am. 90: 844-847.
Martin, S.J., M.E. Correia-Oliveira, S. Shemilt, and F.P. Drijfhout 2018. Is the salivary gland associated with the honey bee recognition compounds in worker honey bees (Apis mellifera)? J. Chem. Ecol. 44: 650-657.
Noonan, K.C. 1986. Recognition of queen larvae by worker honey bees (Apis mellifera). Ethology 73: 295-306.
Page, R.E. Jr. and E.H. Erickson Jr. 1984. Selective rearing of queens by worker honey bees: kin or nestmate recognition. Ann. Entomol. Soc. Am. 77: 578-580.
Page, R.E. Jr., R.A. Metcalf, R.I. Metcalf, E.H. Erickson Jr. and R.L. Lampman 1991. Extractable hydrocarbons and kin recognition in honey bee (Apis mellifera L.). J. Chem. Ecol. 17: 745-756.
Szabo, T.I. 1974. Behavioural studies of queen introduction in the honey bee 2. Effect of age and storage conditions of virgin queens on their attractiveness to workers. J. Apic. Res. 13: 127-135.
Szabo, T.I. 1977. Behavioural studies of queen introduction in the honey bee 6. Multiple queen introduction. J. Apic. Res. 16: 65-83.
Yadava, R.R.S. and M.V. Smith 1971. Aggressive behavior of Apis mellifera L. workers towards introduced queens II. Role of mandibular gland contents of the queen in releasing aggressive behavior. Cand. J. Zool. 49: 1179-1183.
Clarence Collison is an Emeritus Professor of Entomology and Department Head Emeritus of Entomology and Plant Pathology at Mississippi State University, Mississippi State, MS.
]]>Click Here if you listened. We’re trying to gauge interest so only one question is required; however, there is a spot for feedback!
Read along below!
Found in Translation
Sweet and Sour Honey
By: Jay Evans, USDA Beltsville Bee Lab
There are many ways that honey bees improve our diets but honey consumption was an early reason to wrangle this species. The taste for honey persists today around the world, sustaining sideliners, families and large corporations in many parts of the world. It is also widely known to soothe and improve relations with neighbors, in-laws and bosses. With any high-value product, there is a risk of inadvertent or purposeful false advertising.
One honey quality trait that is easy to control is water content. Small-scale beekeepers routinely put their honey crops and relationships at risk by bottling honey that hasn’t been fully processed by bees to a net water percentage under 19%. Watery honey both feels weird and is prone to unintended fermentation. Choosing properly capped frames goes a long way to eliminating this problem. If you live in a humid place like Maryland, there is also some risk that open honey will dehumidify some of the local air, pushing water content back above dangerous levels. Truly dry honey can be achieved by technique and awareness, but if you are curious and want to directly assess the water content of your crop, Hanna Bäckmo gives a nice review of the styles and costs of refractometers used by beekeepers in this magazine (https://www.beeculture.com/refractometer/). Certainly, steady honey producers would benefit from investing in, and calibrating, these things.
A bit out of reach for most of us, but essential for the industry, are lab-based assays aimed at confirming honey purity. The methods used for this continue to improve, putting clumsy or sneaky honey producers on notice. Notably, honey yields can be stretched by a variety of refined or expelled sugars. This might be inadvertent, when syrup fed by beekeepers in the Fall for Winter survival lingers, capped until Spring. There is no easy answer to this, certainly not from me, but step one is to get bees through Winter safely, and then assess any remaining capped stores to see if these stores are bona fide honey or syrup that bees dried down but didn’t gobble up as it came in. Ask a beekeeper near you for help.

Photo by Meggyn Pomerleau on Unsplash
More insidiously, producers or packers might outright add less expensive fillers to their honey, increasing yields but losing some of the magic of honey. The technology used to detect such adulteration is improving, and several techniques are now used by regulators, producers and packers to make sure honey is pure. The International Honey Commission described forensic methods for honey purity nearly 30 years ago and updated these methods in 2009 (https://www.bee-hexagon.net/english/network/publications-by-the-ihc/). The U.S. Food and Drug Administration, keeping honest folks honest across the industry, regularly tests new methods against imported and domestic honey to identify so-called ‘economically motivated adulteration’. Using a well-established technique, Stable Carbon Isotope Ratio Analysis (SCIRA), the FDA recently screened bulk and bottled honey samples from eight countries whose honey is imported into the U.S. (https://www.fda.gov/food/economically-motivated-adulteration-food-fraud/fy2122-sample-collection-and-analysis-imported-honey-economically-motivated-adulteration). This test distinguishes ‘C4’ plant sources (largely grasses and grains) from ‘C3’ sources (all the plants with prettier, bee-visited, nectar-rich flowers). The test simply asks if the unexpected C4-sugars, often from corn syrup or sugar cane, are over-represented in honey. There is some tolerance of these C4 sugars due to bee management or assay imprecision but that level is quite low, maybe 7% by volume. Each country in the FDA screen had at least one suspicious honey batch, but the overall frequency of such batches was 10%, a level roughly similar to a much larger recent study in Europe and indicative that honey, by and large, is as advertised.
There are several newer techniques in play now for the high-stakes race between regulators and those who might diminish the reputation of honey. Dilpreet Singh Brar and colleagues in A comprehensive review on unethical honey: Validation by emerging techniques (Food Control 2023, 145, 109482, https://doi.org/10.1016/j.foodcont.2022.109482) describe nearly 50 ways to test your clover. Within the alphabet soup of available methods, they reveal six chromatographic platforms (basically methods to separate parts of a whole by size, electric charge or affinity to some sort of ‘bait’) with increasing sophistication. These machines should put fear in anyone whose honey is not perfectly sound.
As a geneticist, I am fascinated with so-called environmental DNA (eDNA) screens, whereby a complex soup is scrutinized for the genomes of the diverse organisms floating in it. Many will remember the application of eDNA screens worldwide to identify levels and variants of the SARS-Cov-2 virus in city and town wastewater systems (poor interns!; https://www.nih.gov/news-events/nih-research-matters/tracking-sars-cov-2-variants-wastewater). This same methodology is now widely used to confirm the botanical sources of honey, the genotypes of the bees collecting that honey and the myriad of other organisms from the hive environment. Practically, this method also precisely identifies any honey contaminant with a biological source, from corn syrup to diverse flower sources mixed in accidentally in coveted monofloral honeys. It is also a sensitive assay for honey bee disease agents.
For the past 20 years, genetic analyses of honey from hives have been used to confirm the presence of the bacterium responsible for American Foulbrood, Paenibacillus larvae. Federico Lauro and colleagues in Rapid detection of Paenibacillus larvae from honey and hive samples with a novel nested PCR protocol (International Journal of Food Microbiology 2003, 81, 195-201, https://doi.org/10.1016/S0168-1605(02)00257-X) showed the value of this technique for keeping track of non-symptomatic P. larvae populations. More broadly, Leigh Boardman and others have confirmed that this technique can provide a snapshot of the whole range of microbes found in colonies (Boardman, L., P. Marcelino, J. A., Valentin, R. E., Boncristiani, H., Standley, J. M., & Ellis, J. D. Novel eDNA approaches to monitor Western honey bee (Apis mellifera L.) microbial and arthropod communities. Environmental DNA. 2023; https://doi.org/10.1002/edn3.419). Here, colony-collected honey is analogous to the worker-bee samples now used in many disease surveys. Honey collections have the added value of pointing out long-ago arrivals, providing a sort of fossil record for the plants and other organisms a colony might have come into contact with during the past year. The genetic methods behind these screens are astoundingly sensitive (remember, viruses floating alone in tons of sewer sludge) and honey or hive-based screens have promise for anything from virus outbreaks to the detection of newly invasive mites and other pests. It is incredibly hard to pass through an environment without shedding a little DNA, and a little goes a long way for these sensitive methods.
Economically motivated adulteration is detectable with some effort and that’s a good thing for all of us. Honey screening, especially with the twist of identifying genetic signals from hive organisms, is also becoming a nice tool for scientists keen on monitoring disease, plant sources and the genes of the bees that did all the work.
]]>Click Here if you listened. We’re trying to gauge interest so only one question is required; however, there is a spot for feedback!
Read along below!
Found in Translation
Gut Microbes Help Bees Survive the Season
By: Jay Evans, USDA Beltsville Bee Lab
It will surprise most Bee Culture readers that microbes come in flavors that can be good, bad or indifferent to the health of their honey bee hosts. As we approach Fall, it is tempting to focus on the microbes on the good side and try to find out how to feed them for bee health prior to Winter. As someone who studies honey bee disease, I can’t help but focus on the good microbes that might interfere with agents of harm lurking in our beehives.
Kirk Anderson and colleagues in the USDA’s Tucson Carl Hayden Bee Research Laboratory have been exploring the impacts of gut microbes on bee health for a decade now. In past work, they showed how these microbes are beneficial in the guts of bees but generally ‘don’t’ help in the processing of pollen stored as bee bread. They have also shown how queens and workers differ greatly in the microbes they harbor and the impacts of bee contact on moving microbes around (see Anderson’s ‘Google Scholar’ profile for lists of his papers on these topics; https://scholar.google.com/citations?user=JiEFFkIAAAAJ&hl=en&oi=ao).
They have also explored how bees suffer mortality when the delicate microbial balance is upset. Recently, they have investigated honey bee overwintering, testing for the right mixes of nutrition and temperature that improve the odds of colony survival (hint: cold is good, to a certain degree). In a paper this past year, they describe how the gut microbes of bees react before and during Winter, building the case that microbes are critical for overwintering success (Anderson, K.E.; Maes, P. Social microbiota and social gland gene expression of worker honey bees by age and climate. Scientific Reports 2022, 12, 10690, doi:10.1038/s41598-022-14442-0).
They also show that the overwintering environment can favor certain microbes that are less helpful for bee health. Specifically, bee colonies overwintered in a warm environment started with the typical population of gut bacteria but that population broke bad in the end, notably thanks to overgrowth (these were NOT found in bees) as well as several types which ARE known to decrease bee health. Just what it is about warmer Winter environments that favors an odd, and apparently harmful, bacterial group is not known, and solving this will be key in future work aimed at prepping bees for current or future Winter climates.
More generally, disease agents are opportunists; taking advantage of their victims when something else is out of whack. These opportunities can arise from stressors in the environment, poor genetics or inadequate nutrition. Opportunities might also arise when populations of good bacteria are somehow absent. There are a myriad of ways that such ‘good’ microbes could help bees in the face of disease, from providing a physical layer on the gut wall that frustrates pathogens, to improving nutrient transfer or stimulating bee immunity.
Finally, gut microbes might directly attack the bad actors. Studies showing increased honey bee disease following heavy antibiotic treatments provide ample evidence for the roles of natural bee bacteria. In one such study, led by Jiang Hong Li and my USDA colleague Judy Chen (Li, J.H.; Evans, J.D.; Li, W.F.; Zhao, Y.Z.; DeGrandi-Hoffman, G.; Huang, S.K.; Li, Z.G.; Hamilton, M.; Chen, Y.P. New evidence showing that the destruction of gut bacteria by antibiotic treatment could increase the honey bee’s vulnerability to Nosema infection. PloS one 2017, 12, e0187505, doi:10.1371/journal.pone.0187505). Gut microbes were shown to help bees resist nosema disease. A cleansing of gut bacteria by an intensive antibiotic regime resulted in shorter lifespans overall, and increased the impacts of nosema exposure on longevity.
Sean Leonard and colleagues, in the University of Texas laboratory of Nancy Moran, showed that a human assist can further sharpen the impacts of natural gut microbes on bee parasites. Specifically, they engineered (in the laboratory) a common ‘good’ bacterium of bees so that it targeted challenges as distinct as Varroa mites and Deformed wing virus (Leonard, S.P.; Powell, J.E.; Perutka, J.; Geng, P.; Heckmann, L.C.; Horak, R.D.; Davies, B.W.; Ellington, A.D.; Barrick, J.E.; Moran, N.A. Engineered symbionts activate honey bee immunity and limit pathogens. Science 2020, 367, 573-576, doi:10.1126/science.aax9039).

Nosema. Credit: Qiang Huang
Work this year based on the same strategy (led by Qiang Huang from Jiangxi University, working in Moran’s lab) showed resident bacteria could be altered to successfully target nosema disease (Huang, Q.; Lariviere, P.J.; Powell, J.E.; Moran, N.A. Engineered gut symbiont inhibits microsporidian parasite and improves honey bee survival. Proceedings of the National Academy of Sciences 2023, 120, e2220922120, doi:10.1073/pnas.2220922120). Bees with the engineered bacteria both lived significantly longer and had far fewer nosema spores to pass on to their nestmates. Interestingly, bees fed the gut bacterium alone, and the bacterium with a nonspecific (not targeting nosema), modification also showed signs of reducing disease impacts, supporting the evidence that the bacterium itself is also a friend to bees.
Short of this high-tech solution, are there ways that beekeepers can help nurture the natural gut bacteria found in their beehives? If you supplement your bees, a recent paper by Elijah Powell and others in Moran’s group suggests that pollen-based supplements tend to lead to a more balanced ‘core’ set of bacteria in the bee gut, possibly decreasing the threats from at least one bacterial pathogen of adult bees (Powell JE, Lau P, Rangel J, Arnott R, De Jong T, Moran NA (2023) The microbiome and gene expression of honey bee workers are affected by a diet containing pollen substitutes. PLoS ONE 18(5): e0286070. https://doi.org/10.1371/journal.pone.0286070). I know there are many colony supplements available and I don’t claim this makes a pollen-based supplement better for bees overall than supplements with a different protein source (nor, of course, does this represent any formal endorsement of one type of bee feed over another). Still, it is interesting to contemplate how particular supplements affect not just bees but the hitchhiking microbes that have adapted to life in their guts.
One thing is clear from these diverse studies. While many of us focus on the microbes whose effects are damaging to bee colonies, most hive microbes are neutral or even beneficial to their bee hosts in Summer and Winter. Bees have been harnessing this power for millennia, and we would do well to help them sustain the right mix of gut partners.
]]>
Dr. Tracy Farone
Technical Updates
By: Dr. Tracy Farone
It is mid-May here in the foothills of Pennsylvania. The locust trees are in full bloom. It looks like it will be a good year for them. “Good for the bees,” says the beekeeper voice in my head. The white-tailed deer have changed color into that beautiful reddish brown that pops out within the fresh, green backdrop of the woods. As my “barn” cat (but not really a barn cat), Sylvester, snoozes, stretched out at my feet, I just watched a doe trot away from a salt block 20 yards from my deck. I am a couple of days out from the end of the semester, time to take a breath…The last thing I want to think about is meetings, committees and the possible political acrobatics that go along with them.
I must admit I usually really hate meetings… “analysis paralysis,” pre-determined “communication,” hours of my life I will never get back, things “old” people do, and such. I have always thought it ironically funny that “committee” is the term for a gathering of vultures. But I am also appreciating the importance of voicing and hearing different perspectives on issues and how it’s extremely important in today’s world. And those that step up and serve on organizational committees are giving up their valuable time to contribute to important and ever on-going work.
As promised, I would like to give you an update and summary on a few exciting collaborations that have recently taken place and hopefully bring about positive relationships and outcomes between the beekeeping industry and veterinarians. The American Veterinary Medical Association’s (AVMA) Animal Agriculture Liaison Committee (AALC) Meeting was held at AVMA Headquarters in Schaumburg, IL May 3-4, 2023. I had the opportunity to be a “fly on the wall” at times as an alternate delegate via ZOOM for some of the meeting. The Honey Bee Health Coalition’s (HBHC) Annual Meeting in Sacramento, CA was held at the same time. Both meetings hosted veterinarians representing honey bee medicine for the FIRST time. All representatives were veterinarians also serving on the Honey Bee Veterinary Consortium (HBVC) board.
 The American Veterinary Medical Association’s (AVMA) Animal Agriculture Liaison Committee (AALC) Meeting Summary:
The American Veterinary Medical Association’s (AVMA) Animal Agriculture Liaison Committee (AALC) Meeting Summary:
I have been an alternate delegate representing honey bees on this committee for four to five months now. I am still trying to figure out the ropes, doing mostly listening (a benefit to being the alternate). I can say the committee is continually active with legislative consulting and policy considerations coming to my email box every other day. I can also say that the committee is absolutely enthralled to learn more about honey bees. As an alternate, I did not attend the meeting in person, but Dr. Terri Kane was there, near Chicago, representing. I jumped into the meeting via ZOOM when I could. Some other perspectives include those that represent veterinarians and producers in the areas of veterinary pharmacology, bovine, fish, aquatics, swine, small ruminants, sheep, public health, cattle, chickens, turkeys and the reproduction of animals, as well as government entities like the FDA and USDA.
Discussions include topics like, the Farm Bill; various drug regulation bills; protective measures for maintaining a safe food supply; humane guidelines in animal handling; policies for identifying, preventing, and controlling several current disease threats; and reports on current issues affecting each industry represented and any on-going actions in place. Our honey bee report included information on the progress made within the HBVC and multiple Colleges of Veterinary Medicine to increase honey bee related education of veterinarians and veterinary students to better serve the industry through grant projects, additional curriculum and certification programs for practicing veterinarians. I wish I could get into more detail, but I am bound by a non-disclosure agreement and a secret handshake (just kidding about the handshake). Maybe I will work on the handshake when I attend a meeting in the flesh.
 The Honey Bee Health Coalition’s (HBHC) Annual Meeting Summary:
The Honey Bee Health Coalition’s (HBHC) Annual Meeting Summary:
The stated purpose of the HBHC annual meeting is to “advance dialogue and action across workstreams in the priority areas of forage and nutrition, hive management and crop pest control.” Focuses included almond production, bee protection, The Bee Integrated Demonstration Project and building relationships within members. Drs. Kristol Stenstrom and Britteny Kyle represented veterinarians and the HBVC, a new member of the HBHC, again for the first time. Various reports were shared on the status of honey bees, pollinators and the industry from both the agricultural and conservational perspectives. Best practices and projects involving disease management, habitat management and pesticide use were working topics of discussion.
Next on the List: Euthanasia and Depopulation Procedures in Honey Bees.
The AVMA is extremely interested in learning more about recommendations and guidelines for euthanizing honey bee colonies in various situations, in the safest and most humane manner. Various situations include smaller verses larger operations, stationary hives, migratory hives, emergency de-population procedures, euthanasia for public safety reasons and euthanasia for disease mitigation reasons. AVMA recommendations and guidelines exist for nearly every type of animal that veterinarians work with, except honey bees. I have been asked to be part of a special sub-committee to consider, write up and present recommendations and guidelines to the AVMA. As we begin this work, I am open to reader’s suggestions on the topic. Oh boy… another committee, here we go!
Click Here if you listened. We’re trying to gauge interest so only one question is required; however, there is a spot for feedback!
Read along below!
Found in Translation
City Bee, Country Bee
By: Jay Evans, USDA Beltsville Bee Lab
In Aesop’s fable, City (Town) Mouse, Country Mouse, a city mouse regales her skeptical country cousin with a rosy view of high density living. Sampling both, the country mouse prefers to stay put, largely because “the country mouse lives in a cozy nest at the bottom of a tree. Her home is small, but it is warm and comfortable.” Plus… no cats!
Beekeepers and bee scientists like to contrast the lives of bees under our care in apiaries (dense cities of colonies) versus those out on their own in trees. Aside from giving general insights into bee biology, these comparisons can predict the risks of managed and feral bees sharing disease while also showing how well ‘city’ and ‘country’ bees deal with various stresses. We have great data for the numbers of managed colonies, but how many country bees are we talking about?
I have discussed before the achingly beautiful (and hard) work by Tom Seeley and students assessing feral bees in a U.S. forest. Borrowing from those and similar studies, we can get a rough estimate of how many country bees there are in hollow trees and other cavities. My Sunday afternoon and small brain can’t grapple with honey bee density in deserts and the vast tundra, but considering four adjoining states (New York, Pennsylvania, Maryland and Virginia) with decent land-use data from the USDA (https://www.ers.usda.gov/data-products/major-land-uses/maps-and-state-rankings-of-major-land-uses/), we can estimate ‘suitable’ acreage (fallow fields, pasture and forests) at around 58 million acres total (60% of the available land). Using consensus estimates of 2.5 colonies/square-mile (one colony/square kilometer, 0.004 colonies/acre), one arrives at 233,000 feral honey bee colonies in these four states. According to USDA (https://www.nass.usda.gov/Surveys/Guide_to_NASS_Surveys/Bee_and_Honey/) ,there were 67,500 managed colonies in these states on January 1, 2021, surveying beekeepers with five or more hives. Even doubling this number to account for backyard beekeepers and those who evade surveillance, there are still fewer managed than feral colonies in these regions.
 So, free-living bees are likely to be important for their own sake, and for the environment. What’s it like out there? Taking a disease angle, several studies have compared the relative disease loads of managed and feral colonies in the U.S. Amy Geffre and colleagues from San Diego sampled boxed and free-living colonies (three colonies each) seven times over the course of a year to measure virus levels for three common bee viruses (Preliminary analysis shows that feral and managed honey bees in Southern California have similar levels of viral pathogens. 2023. Journal of Apicultural Research, 62:3, 485-487, DOI:10.1080/00218839.2021.2001209). Both colony types were remarkably similar in virus levels, changing with the season but hardly differing from each other.
So, free-living bees are likely to be important for their own sake, and for the environment. What’s it like out there? Taking a disease angle, several studies have compared the relative disease loads of managed and feral colonies in the U.S. Amy Geffre and colleagues from San Diego sampled boxed and free-living colonies (three colonies each) seven times over the course of a year to measure virus levels for three common bee viruses (Preliminary analysis shows that feral and managed honey bees in Southern California have similar levels of viral pathogens. 2023. Journal of Apicultural Research, 62:3, 485-487, DOI:10.1080/00218839.2021.2001209). Both colony types were remarkably similar in virus levels, changing with the season but hardly differing from each other.
In Persistent effects of management history on honey bee colony virus abundances (2021. Journal of Invertebrate Pathology 179:107520, https://doi.org/10.1016/j.jip.2020.107520), Lewis Bartlett and colleagues found similar patterns between free-living and managed colonies but noted that the style of management might play a role. Namely, colonies maintained in a larger commercial apiary (hundreds of colonies) tended to have the highest levels of most viruses, with feral and low-intensity ‘backyard’ colonies being about the same. As in most field studies, there is abundant variation for viral disease within each category, so these results will need even more sampling to see how viruses and bees fare under different management styles. Nevertheless, they suggest that beekeepers adopting a ‘country bee’ approach by spacing out colonies to reduce urban interactions will be doing their bees a favor.
In the most ambitious study to date, Chauncy Hinshaw and colleagues surveyed 25 colonies each from feral and managed colonies in Pennsylvania (2021. The role of pathogen dynamics and immune gene expression in the survival of feral honey bees. Frontiers in Ecology and Evolution, 8, 594263. https://doi.org/10.1080/00218839.2021.2001209). They surveyed ample bee numbers per collection (75 worker bees), perhaps getting a better sense of average disease loads. Even better, they paired similar city and country colonies from a bunch of regions, which helps account for other factors that might change virus loads. In this study, managed colonies tended to have lower levels of mite-transmitted deformed wing virus, presumably reflecting mite treatments, and roughly similar levels of black queen cell virus and nosema. Perhaps reflecting pathogen exposure, feral colonies had higher levels of several immune response proteins as well. Given the higher number of sampled colonies, these researchers were also able to show how their measurements related to colony fates. As in prior studies, deformed wing virus, presumably alongside mite loads, was a good predictor of a bad colony outcome.
Colonies showing higher levels of two immune genes, once other factors were evened out, were more likely to survive the study period. Arguably, these proteins might be good predictors of genetic components that help bees survive in the face of disease.
More can be done to contrast the lives and successes of city and country bees. These comparisons can help improve bee management by those of us keeping bees in clusters of Langstroth high-rises. It is also fun to think of bees in the ancestral habits they have followed for thousands of years. Country bees almost certainly have more threats now than they did when humans were more scarce, and there has to be some level of contact between city bees and country bees that muddies all of these comparisons, but in many ways the presence of country bees at all is comforting. Left to their own care, they are making country homes work wherever they can, and that is a good lesson for beekeepers.
In full disclosure, the lives of country bees were not on my mind until a recent inquiry from British bee researcher Francis Ratnieks and his graduate student Ollie Visick. In their Laboratory for Apiculture and Social Insects (https://www.sussex.ac.uk/lasi/), they are comparing the lives of free-living honey bees in their native range to their hived cousins. As ecologists, their studies will give insights into how honey bees used to live in the forests and fields of England. I thank them for the prompt (and welcome hot tips from any of you) and look forward to reading their results!
]]>New(ish) Beekeeper Column
By: Richard Wahl
We all know that one of the greater challenges in beekeeping is shepherding bees through our variable northern Winters. But what other weather factors should we consider when we are planning our beehive inspections, splits, mite control or feeding regimens?
In my experiences with beekeeping, I have come to rely on signals from nature and the variable weather patterns in my own surrounding environment rather than reliance on specific calendar dates that follow the same schedule year after year. So in this article, I will relate some of the clues and weather events that signal the appropriate time to take certain actions that have resulted in my success in getting at least one hive through every Winter for the past thirteen years. My greatest result occurred several years ago with nine hives going into Winter and those nine hives successfully surviving through the following Summer. This result allowed me to sell a few nucleus hives (nucs), raise a few more queens and take another step toward being a self-sustaining beekeeper.
As the Year Starts
At the beginning of the year, shortly after Christmas, is when I briefly open hives to add a cane sugar food supplement to the hive. It seems that every so often around the Christmas/New Year’s holidays there is a day or two that gets above 45°F (7°C) that allow for both cleansing flights and the insertion of extra food supplies. There are various ways to supply additional food resources including hard candy boards, sugar patties or granulated sugar over newspaper. I hesitate adding any sugar source earlier than late December. Any form of cane sugar is harder for the bees to digest and if they decide to tap into the additional hard sugar source in the Fall, it can possibly result in a form of dysentery. Dysentery is often the result of bees not being able to leave the hive for cleansing flights and finding it necessary to relieve themselves in the hive. The February and March time frames are when most hives are lost over Winter due to a lack of food resources.

Winter sugar over parchment paper is nearly used up, the remains of a partial pollen patty at lower left of sugar.
Another Winter task is that every other month or so I will also use a bent ½ inch metal bar to clean out any dead bees from the bottom board. Once temperatures only occasionally drop below freezing at night, I will also remove my insulation sleeves that cover all but the bottom and top entrances to the hives. Some of my fellow beekeepers use blanket insulation and also remove them when temps begin to only erratically fall below freezing at night.
My next clue is the budding of maple trees in my yard. In some years, I have seen white pollen brought into hives in very early February; possibly from pussy willow shrubs, but not of sufficient quantity to support the needs of potential new larva. If one does see pollen being brought into the hive, this is a clue that the hive is most likely healthy and the queen has started to lay eggs, although in very small quantities this early in the year. In late March or early April there is one of a dozen maples in my yard that is always first to have the buds pop open. On a warm, sunny day, standing under the tree, it sounds like you are standing in a beehive. I use this as my signal to check the cane sugar supply once again and add a partial pollen patty to each hive.
The next pollen/nectar flow will not occur until a month later, in late April or early May. If they gather enough pollen from maples and other sources they will not use much of the pollen patty. But if rainy, cold weather precludes much pollen collection they may use most, or all, of the supplied patty and it may even need to be replaced before the dandelion bloom.
Heavy dandelion bloom is the next signal I use to know that Spring flowers and the dandelions are providing the first nectar flow. This is also my signal to do my first deep hive inspection and commence with any splits I may wish to do. Moving frames around, even if exactly replaced before dandelion bloom, can disrupt the hive in such a way that the cluster does not reform to provide the needed warmth for new eggs and larva resulting in the loss of the hive.

Bent metal bar used to clean out bottom board in Winter.
Opening a hive has a different meaning than inspecting a hive. Up to this point, I have only opened the top of hives to add sugar or pollen patties, while inspecting means to quickly examine each frame as it is removed and replaced or substituted if doing a split. The methodology of splits was covered in the April issue so I will not repeat my split techniques here. This is also the time where I will clean off the bottom board and remove excess old or pollen saturated frames.
Once May arrives, the beekeeping season gets into full swing here in SE Michigan. It is a good time to do the first mite check and initiate treatment, if called for. It is suggested that for the first few months of beekeeping the new beekeeper check hives once a week to every ten days. This is also a good recommendation for any new hives or nucs that have been started in order to monitor their progress. These do not have to be deep hive inspections looking at every frame. Often starting an inspection by pulling a frame or two from one side until eggs/larva are spotted is enough to see the hive is functioning well with an adequate queen without ever seeing the queen or looking at every single frame. As your comfort level and knowledge increases, hives may not need to be inspected for a month or more if things look normal with bees coming and going. Bees that are bringing in some pollen is a good sign there is a laying queen and larva to be fed.
Weather Affects Flying Time
Since beginning beekeeping, I find I keep a much closer watch of weather forecasts to determine the best times to work with my hives dependent on weather. As the Summer flowers start blossoming and nectar flows get into full swing, weather is the key factor in how much time bees can be flying and making collections of nectar, water, pollen or propolis. Any new splits or weaker hives can benefit from a feeding of one to one sugar syrup and an initial mite treatment if needed. I like to use a single Hopguard strip in five frame starter nucs just as a precaution. From this point on through the Summer, it is a matter of periodically checking hives to be sure the queen is laying, mite loads do not become excessive and no inherent diseases occur. When all but one or two frames in the top most super are drawn with comb and filled with brood or nectar and honey it is time to add another super. I prefer to keep my bee’s brood chamber in two ten-frame deeps with a queen excluder under any honey supers that are continually added through the Summer. I know of area beekeepers that work with eight frame medium supers and use three supers as their brood chamber with equal success. If I were to start over again, I would most likely choose the eight frame triple supers due to the weight factor of a ten frame deep super when full.
Taking weather into consideration, there are factors that come into play when the bees will be less agitated when doing an inspection. It is generally recommended that inspections be done on days when the outside temperature is above 55°F (13°C). On a warm, sunny day, most of the foragers will be out of the hive. If there is a front moving in or it is rainy out, the bees seem to be able to sense this and will be more agitated. Likewise, cloudy or windy days are not optimal times for inspections. The time of day that works the best seems to be between 11:00 a.m. and 3:00 p.m., although on nice Summer days that are longer, inspections can stretch into the late afternoon or early evenings.
When opening a hive, listen to the noise of the bees. If it goes from a peaceful hum to a louder roar it may not be the best time for a deep inspection of all frames. I recall helping a new beekeeper several years ago who had a work schedule that allowed for inspections to only occur on weekends. Several months of rainy or windy weather made it quite difficult to inspect during optimal weather which made for a more difficult beekeeping Summer. The hobby beekeeper with other employment challenges may find it difficult to find optimal overlaps between good weather and their free time to inspect hives.
Most of my reading and research indicates that mite checks are recommended about every month to month and half with the most critical time being August through September. This is when the mite population is exploding just as the bee population begins to decrease in preparation for Winter. Mite population control is without question the current most important part of beekeeping to insure hive survival over the coming Winter. When doing splits, I insert drone frames which forces me to get into new hives in less than 24 days for their removal. This assumes drone comb has been drawn, capped and drone brood is present. Mites prefer drone brood due to the slightly longer 24 day period it takes for drones to emerge. Removing drone frames prior to 24 days precludes a mite explosion as drones emerge from cells. When mite counts warrant treatment, I follow with a formic treatment in late June or early July followed by another treatment in late August or early September and finally one or two oxalic acid dribbles in October and late November if needed.
Harvesting Honey
Fully capped frames of honey can be taken any time of the beekeeping Summer/Fall season. I have taken honey from remaining Spring hives where the bees did not survive the Winter. If doing this, it is easiest if the honey frames are warmed and checked for any crystallization. Extracting frames that are partially crystallized can quickly plug up the filtration screens and make it very hard to strain the resultant honey. I have found it much easier to feed any unused overwintered frames back to the bees or use them in new hives or nuc splits. Bees from active hives will soon find frames that are set out some distance away from the apiary and will remove the surplus honey to existing honey supers. I have also had some luck with a partial super of near full frames placed over an inner cover that is on top of the upper most honey super allowing bees to clean out the excess frames of honey. During my first few years of beekeeping I only collected honey once in the Fall. This sometimes resulted in very tall hives as supers were added to give the bees more space.

Three deeps and four full honey supers with a fifth added before Fall honey harvest reached over six feet and resulted in future harvests occurring twice a season.
I have since decided it is easier to make a harvest in late July followed by another in September. Any Fall flow is left for the bees to backfill the brood chamber for their Winter honey supply. If new nucs or hives are made from splits, those new starts may not produce any excess honey for the beekeeper in their first Summer. Taking too much honey from the bees in their first season is also a reason for Winter loss as this may result in Winter starvation.
The amount of nectar the bees collect that can be turned into honey is directly related to weather conditions. Continual rain and thunderstorms during a peak nectar flow can significantly cut down on flying time and wash away available flower nectar. Dearth periods where there is no rain for weeks also effects nectar availability, as the plants are using available ground moisture to sustain leaves and growth rather than producing pollen and nectar for flowers and seeds. Weather that is hotter than normal or nights that are colder than normal also impact the amount of nectar that plants produce. As the beekeeper learns to keep a close eye on weather and forecasts, they can better determine optimal times for inspections and if there will be a larger or smaller honey harvest.
Another aspect to consider is when to start nucs for overwintering. I have found that nucleus hives of four or five frames are best started in May or June but no later than the beginning of July. Four frame nucs started in those months may need a second or even a third story four-frame super added to make space for the increasing number of bees. The earlier the start, the more frames that may need to be added. In the following Spring, five frame nucs can be sold and excess frames used to begin new hives or nucs or simply used to increase ones hive count.
Fall Weather Clues
As Fall weather temperatures get cooler and daylight time gets shorter, the bees will be out foraging less and Fall nectar flows are sometimes questionable. Hives that have had their last honey harvest may benefit from Fall feeding of 2:1 sugar syrup. Any extracted honey supers can be placed over the inner cover and under the outer cover such that only the bees in the hive can clean out the honey supers for storage and reuse the following year. There is less chance of bees storing additional nectar/honey in the extracted honey super if it is placed on a different hive than that from which it was taken.
As the temperatures start to drop below 40°F (4.5°C) at night, it is time to combine hives or restrict hives to smaller spaces. This will also be when the Fall flowers such as golden rod and wild purple asters have passed their peak. If there are several weak hives they can be combined using the newspaper method between supers and pinching the weaker queen. Although, I have had very heavily populated hives come through the Winter in three deeps, I like to confine bees to only one or two ten-frame deeps as Winter approaches. New hives or swarms caught earlier in the Summer are usually best if confined to one ten-frame deep while established hives with a large population of bees may be better if allowed to have two deeps.
As stated earlier, August and September are critical months to keep mite counts under control. A day or two after doing a mite treatment, another test for mites is highly recommended to see if that treatment had an effect. If mite counts are still higher than recommended (3 per 100 in Fall) another treatment may be needed. High mite counts during these months are a strong indicator that the hive may not survive the Winter. As temperatures start to dip below freezing at night, it’s time to winterize the hives. I use a combination of a coroplast sleeve over the sides of the hive as well as Vivaldi style spacers for ventilation over the inner cover.

Coroplast plastic sleeves over hives.
There are other numerous ways to insulate a hive, such as using tarpaper or hive blankets if Winter temperatures can get very cold or are somewhat variable in your area. And this brings us full circle to the beginning of the next year.
As you become more experienced as a beekeeper, noting the changes in nature can lead to more efficient beekeeping dependent on your environments weather conditions rather than on calendar dates. I have found that keeping good notes has helped me improve from year to year. If you are not in a note taking mood, I have included a checkoff page (Download PDF) that can be copied and used as you inspect your hives. This is a slightly modified checklist obtained from a local beekeeper and used with permission from Jim Ford, who works with a Boy Scout troop to obtain various merit badges including beekeeping. Using clues from how weather patterns effect nature in your local environment can lead to a better beekeeping experience.
By: Jerry Hayes
Lots of colony losses once again in 2023. There are three words I want you to remember: Varroa, Varroa, Varroa. And disappointingly, the majority of the beekeeping industry is still not using the Honey Bee Health Coalition vetted, accurate and usable Tools for Varroa Management Guide.
Varroa mites and the Varroa Virus legacy will KILL your honey bees.
In order to be a good manager of your honey bee colonies and reduce/stop losses from Varroa/Virus you, the beekeeper, need to be on your ‘game’ and be a Beekeeper not a Bee-haver.
The Honey Bee Health Coalition (HBHC) has the developed the key educational outreach tool for Varroa control titled, Tools for Varroa Management, A Guide to Effective Varroa Sampling & Control. The latest edition can be found at https://honeybeehealthcoalition.org/wp-content/uploads/2022/08/HBHC-Guide_Varroa-Mgmt_8thEd-081622.pdf. It is based on Federal and State registered, legally approved products which require beekeepers to ALWAYS following label directions. This is all you really need to successfully manage for Varroa control in your colonies. To get you started, we will share some overview of what you need to think about and actually do.
In the Tools Guide each product will have the following individual points in a table: Name, Active Ingredient, Formulation, Route of Exposure, Treatment Time/Use Frequency, Time of Year, Registrant-reported Effectiveness, Conditions for Use, Restrictions , Advantages, Disadvantages, Considerations and a link to a Use Video.
Here we are only going to share Name, Active Ingredient and Conditions for Use, to get you started.
INTEGRATED PEST MANAGEMENT (IPM) is a set of proactive, control methods that offer beekeepers the best “whole systems approach” to controlling varroa. See Tools Guide, pages 6-12.
ESSENTIAL OILS
Tools Guide pages 19-20
Name – Apiguard and Thymovar
Active Ingredient – Thymol
Conditions of Use – Temperature range restrictions: Apiguard – above 59°F and below 105°F (15°C to 40°C), Thymovar: above 59°F and below 85°F (15°C to 30°C).
Name – ApiLife Var
Active Ingredients – Thymol (74.09%), Oil of Eucalyptus (16%), Menthol (3.73%) = camphor ( essential oil)
Conditions of Use – Divide wafer into four pieces and place each piece in a corner of the hive on the top bars. Use between 65°F and 95°F (18°C to 35°C). Ineffective below 45°F (8°C).
NON-CHEMICAL / CULTURAL CONTROLS
Tools Guide pages 26-30
Name – Screen Bottom Board
Conditions for Use – Replace hive bottom; leave space below for trash (‘garbage pit’).
Name – Sanitation (bee biosecurity) comb management
Conditions for Use – Possible negative effect on bee population if five or more combs are moved at one time.
Name – Drone Brood Removal (Drone Trapping Varroa)
Conditions of Use – Only applicable during population increase and peak population when colonies are actively rearing drones.
Name – Brood Interruption
Conditions of Use – Need a queen or queen cell for each split or division created.
Name – Requeening (Ideally with varroa resistant stock)
Conditions of Use – Works best with proper queen introduction methods.
SYNTHETIC CHEMICALS
Tools Guide pages 16-18
Name – Apivar
Active Ingredient – Amitraz (formadine acaricide/insecticide)
Conditions for Use – Place one Apivar strip per five frames of bees. Place strips near cluster or if brood is present, in the center of the brood nest. Only use Apivar in brood boxes where honey for human consumption is NOT being produced.
Name – Apistan
Active Ingredient – Tau-fluvalinate (pyrethroid ester acaracide/insecticide)
Conditions for Use – Temperatures must be above 50°F (10°C). Do not use during nectar flow.
Name – Checkmite
Active Ingredient – Coumaphos (organothiophosphate acaracide/insecticide)
Conditions for Use – Wait two weeks after use before supering.
ACIDS
Tools Guide pages 21-25
Name – Mite-Away Quick Strips
Active Ingredient – Formic Acid (organic acid)
Conditions of Use – Full dose (two strips for seven days) or single strip (seven-day interval then single new strip for an additional seven days) per single or double brood chamber of standard Langstroth equipment.
Name – Formic Pro
Active Ingredient – Formic acid (organic acid)
Conditions of Use – Both treatment options can be applied per single or double brood chamber of standard Langstroth equipment or equivalent hive or equivalent hive with a cluster covering a minimum of six frames. There should be a strip touching each top bar containing brood. Use when outside day temperature is 50°F to 85°F (10°C to 29.5°C)
Name – 65% formic acid
Active Ingredient – Formic acid 65%
Conditions of Use – Use when outside temperatures are between 50°F to 86°F (10°C to 30°C) and leave hive entrances fully open
Name – Oxalic Acid / Api-Bioxal
Active Ingredient – Oxalic acid dihydrate (organic acid)
Conditions of Use – Mix 35 grams (approximately 2.3 tablespoons) of oxalic acid into one liter of 1:1 sugar syrup. With a syringe trickle five milliliters of this solution directly onto the bee in each occupied bee space in each brood box; Maximum 50ml per colony of oxalic acid in sugar syrup; fumigation of two grams per hive in Canada and one gram per hive box in the U.S.; follow label and vaporizer directions.
Name – HopGuard 3
Active Ingredient – Potassium salt (16%) of hops beta acids (organic acid)
Conditions of Use – Corrosive—use appropriate clothing and eye protection. Might stain clothing and gloves.
Click Here if you listened. We’re trying to gauge interest so only one question is required; however, there is a spot for feedback!
Read along below!
Found in Translation
Teaching Bees New Tricks
By: Jay Evans, USDA Beltsville Bee Lab
Bees have innate (think ‘robo-bee’) and learned (‘show me, sister’) behaviors. Recent work with bees has explored the boundaries of these two forms. While it is dangerous to put our own biases on animal behaviors, the complex behaviors measured seem to include ‘play’, ‘puzzling’ and ‘dancing’. Oh yeah, and they can count as well, even showing an awareness of ‘zero’ things, but that was yesteryear’s news from Scarlett Howard and colleagues (Numerical ordering of zero in honey bees, 2018, Science, DOI: 10.1126/science.aar4975).
 What is fascinating about work coming out just this year is that not only do bees show complex behaviors, but they seem to get better at those behaviors by watching their nestmates. Bee dances will be familiar to most beekeepers and students of animal behavior. Successful foragers often tell their sisters where the good stuff is after finishing their foraging flights. Specifically, foragers signal both direction and distance to flower sources using the waggle dance. True to its name, and shown graphically to the right, this dance involves a bee streaking across the comb and shaking its abdomen for the edification of sister foragers. The angle of this dance on a vertical patch of comb signals the direction of a good food source relative to the current position of the sun relative to the hive. The length of each dance streak provides an estimate of the distance to flower patches (or to sugar baits planted by curious naturalists). By repeatedly dancing, they drum up interest and lead future foragers to a better understanding of how far they might have to fly to get these rewards. The discovery of this dance language is decades old, and justified a share of the Nobel Prize in Physiology or Medicine in 1973 for Austrian bee researcher Karl von Frisch. The recent work ups the game by showing that much of this behavior is learned by watching older, more precise, dancers.
What is fascinating about work coming out just this year is that not only do bees show complex behaviors, but they seem to get better at those behaviors by watching their nestmates. Bee dances will be familiar to most beekeepers and students of animal behavior. Successful foragers often tell their sisters where the good stuff is after finishing their foraging flights. Specifically, foragers signal both direction and distance to flower sources using the waggle dance. True to its name, and shown graphically to the right, this dance involves a bee streaking across the comb and shaking its abdomen for the edification of sister foragers. The angle of this dance on a vertical patch of comb signals the direction of a good food source relative to the current position of the sun relative to the hive. The length of each dance streak provides an estimate of the distance to flower patches (or to sugar baits planted by curious naturalists). By repeatedly dancing, they drum up interest and lead future foragers to a better understanding of how far they might have to fly to get these rewards. The discovery of this dance language is decades old, and justified a share of the Nobel Prize in Physiology or Medicine in 1973 for Austrian bee researcher Karl von Frisch. The recent work ups the game by showing that much of this behavior is learned by watching older, more precise, dancers.
Shihao Dong and colleagues set out to study Social signal learning of the waggle dance in honey bees (2023, Science, DOI:10.1126/science.ade1702). Specifically, they judged the dancing skills of self-starters relative to those of bees that were mentored by older, experienced, dancers. To produce a swarm of naïve dancers, they established colonies comprised solely of like-aged bees, so that all bees reached foraging age together and were therefore less likely to benefit from matching the skills of a senior dancer. Bees from these ‘Animal Farm’ colonies were compared to marked bees of the same age which had grown up gazing at the dances of experienced dancers in colonies with a typical age profile. Naïve bees consistently over-stated the distance they had flown to flowers, in effect telling nestmates to fly right past suitable food sources. They also showed more ‘Dance Disorder’ than both older bees and bees that had been exposed to older dancers. Dance accuracy for all dancers improved over time, it just improved much more quickly when bees had older mentors to watch. So what is the lesson here for beekeepers? No, you can’t force your teenager to watch you dance and expect them to get it, but you CAN see how bees in colonies with an abnormal age structure, thanks to rapid premature death of foragers, might continue to slide by spending unnecessary time looking for food. Long-lived bees are those free of chemical stress, raised with adequate protein nutrition, and arguably bees that have avoided mites and other disease. When you protect your bees from these stresses, just think of how their dance lives will improve.
In a study that, for me, deserved two SMH’s, bees were trained to take on puzzle behaviors, or behaviors that simply don’t present themselves to bees when scientists aren’t around. Working with bumble bees, Alice Bridges and colleagues first taught their bees to open small food boxes by pushing on colored (red or blue) tabs. This a behavior I am not sure I could teach my dog, but she is a bit slow. They then checked to see if bees could follow the lead of a nestmate who had already figured out the box trick. While self-learners emerged in the control colonies sometimes got the knack for opening boxes, bees who observed a nestmate open a box were more likely to successfully mimic that behavior. Over time, bees with a teacher opened more boxes, faster, and were rewarded with more sugar treats. Honey bees and some other bee species are known to spontaneously ‘rob’ flowers by chewing directly into nectar pools when those pools are too deep in the flower for their tongues to reach. It would be neat to see if such nectar robbing is also a learned trait, passed on by adventurous foragers who had to learn the trait the hard way. If so, can such teachers target their lessons to their nestmate sisters?
All of these studies push the known boundaries for bee awareness and behavior, showing all the more how lucky we are to have formed bonds with honey bees and other insects. Clever behavioral scientists will no doubt continue to discover profound, and maybe a bit unsettling, awareness by insects. This awareness is likely to be most evident in the highly social honey bees and bumble bees. What’s next, spelling bees? Stay tuned. In the meantime, get out, find a friend and improve your dancing.
]]>By: Becky Masterman & Bridget Mendel

Successful beekeepers track numbers throughout their colonies and apiaries over time. Knowing varroa mite loads might help explain colony death. Honey production yields can reflect habitat quality around your apiaries. Photo credit: Rebecca Masterman
Managing bees is kind of a numbers game. Not in the illegal gambling sense, but instead in the measuring bee health and business sense. New beekeepers are warned about the importance of counting varroa mite numbers in their colonies and hear stories about high percentages of colony loss. Long time beekeepers and many commercial operations remember stories of high honey yields and market prices that are impacted by imports. Whether you are new to beekeeping or a long-time participant, keeping track of key numbers could be good for you, the bees and beekeeping.
It has been said often that beekeeping is local, so your beekeeping numbers should be too. Honey yields per colony vary greatly from state to state (and apiary to apiary within a state and hive to hive within an apiary) as do the pounds of honey that bees need to get through Winter or dearth periods. Tracking key numbers within your state each year can serve as a bee health conversation starter. Let’s hope that the conversations continue each year and are about lower colony losses and higher honey yields.
Here are some numbers that we think are important.
Varroa Mite Loads
Let’s get the mite talk out of the way and address it right away. Varroa mites and the viruses they vector are still one of the greatest threats to the health of your honey bee colonies. Successful beekeepers keep on top of the latest management recommendations and know the threshold where intervention is key to maintaining healthy bees. This threshold might be different than what you think and the threshold changes depending on the time of year. Monitoring mite loads is important. Keeping up on the latest research-based recommendations is also critical to successful beekeeping as your management will change over the years as the threats to your bees change (anyone remember tracheal mites?). This excellent update from the Bee Informed Partnership will bring you up to date on mites as well as provide a link to the latest free edition of Tools for Varroa Management from the Honey Bee Health Coalition. While you are there, we suggest signing up for updates from the Bee Informed Partnership (https://beeinformed.org/2023/03/01/the-importance-of-spring-mite-loads).
Colony Loss Data
Let’s also get the colony loss data out of the way. Following significant colony losses across the U.S. in 2006, the Bee Informed Partnership has been tracking colony losses across the U.S. You can contribute to the survey each year in April by reporting your losses. Reported loss data by states is available all the way back to 2008 (https://research.beeinformed.org/loss-map/).
Honey Market Numbers
The National Honey Board compiles honey data from multiple sources on their website and you can easily spend some time looking at the numbers (https://honey.com/honey-industry/market-overview). For a monthly regional report, Bee Culture’s detailed guide will provide everything you need to know (https://www.beeculture.com/monthly-regional-honey-price-report/).
Honey Yields
If you want to explore your state honey data, the USDA National Agricultural Statistics Service has a searchable database for all things honey and bees, including reported honey yield per colony. You can search national as well as state data across more than 30 years (https://quickstats.nass.usda.gov/).

Measuring available habitat for your bees is difficult but doing so might inspire others to increase floral resources for pollinators. These bees are enjoying the blooming mountain mint planted in the apiary. Photo credit: Rebecca Masterman
Because honey bees are managed pollinators, it is easier to track the numbers described before. One other number that is more difficult to track, yet very important, when talking about honey bee health is Acres of Pollinator Habitat.
How much pollinator habitat is available for bees in your state? It is a difficult number to track as counting the flowers on trees, shrubs, prairies, conservation land, cover crops and more (bee lawns, gardens, roadside habitat, etc.) is not easy, but we love those who are trying to do just that. Please visit Homegrown National Park’s website and spend some time appreciating their efforts to support grassroots conservation with a way for all of us to map our native plantings (https://map.homegrownnationalpark.org/Dashboard/Country).

Becky Masterman led the UMN Bee Squad from 2013-2019. Bridget Mendel joined the Bee Squad in 2013 and has led the program since 2020. Photos of Becky (left) and Bridget (right) looking for their respective hives. If you would like to contact the authors with your number stories or thoughts, please send an email to [email protected].
Knowing the status of food for your bees is important. Beekeepers usually work backwards, as they place their bees and measure the honey. If honey production is low (compare yours to the average honey yield), then it is possible that there are not adequate resources for your bees. If honey production is high, then you are in luck. If you planted flowers specifically for your bees to make honey on, then it isn’t luck, but good planning.
Should we be measuring some of the pollinator habitat in our states? We think it is a great idea and good to know if the numbers are going up or down. Some numbers, like acres planted in CP42 Pollinator Mix in the Conservation Reserve Program are being measured. Is it possible for state or federal agencies to keep track of the habitat they have installed without too much of a record-keeping burden? We don’t know but think we should ask.
Acknowledgments and suggestions:
The authors would like to thank Dr. Marla Spivak for helpful edits and suggestions.
New(ish) Beekeeper Column
By: Richard Wahl
As late Spring moves into Summer and the bee numbers in the hive begin to increase, it is not unusual to have a hive release a swarm. I have seen swarms in our area of SE Michigan begin in mid-May and continue as late as late August. The most frequent occurrence of swarms seems to occur from early June to late July. During my first few years of beekeeping, I would have insisted that these swarms were not coming from my hives. As I have become more aware of the causes for swarming I now believe that in those early years that at least some of the swarms I was catching did indeed come from my hives. But what would cause this natural tendency to swarm and how can it be minimized? The most probable cause for a hive to swarm is overcrowding. Although I knew some of my earlier year hives were strong and felt they had not swarmed, I have now changed my opinion on this point. Just because there still seem to be a lot of bees in the hive after a swarm is not a good indication that the hive did not swarm. An overcrowded hive after a swarm may look, to the new beekeeper, very similar to the pre-swarmed hive even though ⅓ to ½ of the hive may have departed. If a hive is not examined the day before and the day after a swarm it will be very hard to tell if the hive has swarmed. As the hive begins its late Spring and early Summer build-up it experiences a large population increase. By late June or early July, this huge increase may be the precursor to a swarm. If something is not done to preclude this there could be a loss of a significant honey crop later in the season. This is because it has been found that up to 75% of the hive foragers may leave with the swarm. This large decrease in the bees that have reached the forager stage leaves most of the new bees to still go through the nurse bee and internal hive job stages before becoming foragers. This makes sense, since the swarm needs foragers to supply the nectar needs and comb building of a new hive while the bees remaining in the original hive serve as nurse and house bees to support the care of newly emerging bees before becoming foragers. I once had the privilege/misfortune to watch a swarm emanate from a hive and it is an exhilarating and exciting sight to see and at the same time very disappointing. I was about to inspect my hives around 11:30 in the morning. As I was inspecting my most western hive, there seemed to be a lot of activity at the hive entrance of the next hive over. I initially chalked this up to orientation flights as these also exhibit a lot of activity in front of the hive for a short time. But suddenly there was a massive exodus of bees from that next hive as wave after wave of bees marched out the reduced hive entrance and circled around the calm air in a thirty to forty foot circle in front of my hives. After about ten to fifteen minutes the bees coalesced into about a five to six foot diameter circle and departed to the south about ten to twelve feet off the ground never to be seen again. I checked the hive and there were still many bees present. Had I not seen the swarm depart with my own eyes, I would have been none the wiser that this hive had swarmed. It was then I realized some of my earlier year swarms may have been from my own hives. The propensity to swarm is not to be viewed as a bad thing as it indicates the hive was strong to begin with. Hopefully, it will regain its strength as it rebuilds, although this can be a time consuming process. Before I get into some steps that can mitigate a swarm, there are a few other conditions that may be reasons for swarming.

A captured swarm from a backyard pine tree began my beekeeping adventure with no previous knowledge or equipment.
Reasons for Swarming
In addition to the crowding previously mentioned, swarming may also occur due to a heavy mite infestation. As the bee population increases, the potential for mite increase is also present due to the availability of many more capped brood cells in which to reproduce. If the mite population gets out of hand, the bees could decide to swarm to find a better location only taking the phoretic mites with them. Phoretic mites are those riding around on the bee’s backs or thoraxes. Mites in un-emerged cells will be left behind in the hive. Not initiating some type of mite mitigating protocol on one’s hives is the greatest reason for hive losses. Likewise, some method of mite treatment management should take place with a captured swarm to assist the success of that swam in becoming a productive hive.
As mentioned before, not enough space can be a reason for swarming. This is not only true for the crowded double deep hive, but can also happen in as small as a three frame nuc. Small three to five frame nucs need to be watched much more carefully and given added space sooner to preclude the possibility of all frames being filled with nectar and brood almost requiring the nuc to swarm if there is not enough room for more eggs and brood or honey storage space. Over time, brood frames become old and minimally smaller with the pupae cocoons of repeatedly raised new brood in those cells. Additionally, those continually reused cells may retain slight bits of the agrochemicals used in pesticides, which leads to near universal contamination of beeswax in the bee colony. Wax contaminated with these pesticides negatively affects the reproductive quality of queens, drones and the overall quality of worker bees. When the bee’s tolerance of the contaminants in the reused wax brood cells exceeds an unknown threshold the bees may decide to swarm. Therefore, it is a good idea to replace old brood foundation with new frames on a regular basis. I have read that every four to five years is a good time allowance for replacing brood frames but have also seen some respected beekeeper researchers let this reach to an eight to ten year replacement cycle.
Another reason for swarming is the internal hive climate. If the temperature is continually getting too hot or the bees do not have the capability to provide proper ventilation, this may also induce swarming. The bees spend a great deal of effort keeping the internal humidity and temperature at the ideal state around the queen, brood and nectar stores. If drainage or ventilation needs become too severe the bees could decide to swarm.
Yet another reason for the hive to swarm is that there is a problem with the current queen. She may simply have exhausted her ability to lay or fertilize eggs and the hive senses it is time to move on with a new queen.
One final reason for swarming is that it is a natural tendency for all living things to have a propensity for procreation. The simple desire to continue ones genetic stock in future generations can best be accomplished by Apis mellifera with swarming. This inherent drive is almost impossible to identify in order to preclude a swarm by even more experienced beekeepers.

A swarm in flight getting ready to depart the hive area.
Swarming Symptoms
There are a few things the beekeeper can look for in hive inspections that may indicate a precursor to a hive’s potential for swarming. That first, late Spring hive inspection becomes very important in determining a hive’s swarm probability. We all hope for a strong cluster to survive the Winter and become a thriving hive as the first nectar flow begins. If within a month of that first strong hive inspection and there are more than five to seven frames of brood in a double deep Langstroth hive, then it is most likely time to do something to manage that hive. I have found that adding another brood deep or honey super when all but the last frame or two are filled results in a less likely potential for swarming with first time overwintered hives.
The presence of open, unused queen cups along the bottom of frames is normally nothing to be concerned about. These are usually only present for an emergency response if needed. But if those cells become fully developed queen cells, or start to contain larva, the hive may already have decided to swarm.
Significantly reduced activity can be a sign of potential swarming as bees are not bringing much into the hive. This can be hard to ascertain by the new beekeeper and may be caused by lack of space for more nectar or pollen brought into the hive. I find I often need to remove a frame or two of older pollen filled frames as there is such a wealth of pollen sources here in SE Michigan. Often, more frames than needed become nearly saturated with pollen using up valuable space for more brood or nectar stores.
Another indication that a hive is getting ready to swarm is that the hive has had no weight gain in a week or so period. I have to admit that I do not monitor my hive’s weights until the early Winter weight check, if at all. But research has shown that if a hive does not gain much weight during a nectar flow week, it is almost a sure sign of swarm preparation to come. As I became more experienced as a beekeeper, I found that more careful monitoring of my hives could give a good indication of swarming potential. From there I have found that splitting a strong hive early on is the best way to avoid the swarming instinct. In a previous article, I went into the elements of how to split a hive so I will not repeat those steps here.
Mitigating a Swarm
There is a lot written about removing queen cups to preclude the swarming instinct. The thing to remember is that by removing queen cups you are not changing the bee’s instinct to swarm if it already exists. The bees can build new queen cups in a matter of days so removal must be a continual process every two to three days, not an objective most beekeepers prefer to try. The destruction of queen cells has never proven to be a successful method of swarm control. I have seen eggs laid in cells for worker bees that appear to be only ⅓ of their final size. The bees continue to build the cells even as the egg and larva begin to grow. The bees may use this same technique for new queen cells and if an unfound queen cell that was missed emerges the hive may swarm earlier in the normal development of the replacement queen. Destroying queen cells on a second try runs the risk of early swarming and no new queen left for the remaining hive to develop. This could also result in the initial swarm being even larger than if you had not interfered with the removal of queen cells.
The best way to curtail the possibility of swarms is to do a split as soon as a hive is perceived to have swarm characteristics or seems strong enough for a split. A split is actually akin to creating an artificial swarm and the best known way to minimize the possibility of swarming. If developed queen cells or queen cups containing larva are found, these are perfect frames to move to starter nucleus (nuc) hives. A year ago, I split all six of my hives that came through Winter in a strong state with plenty of bees in each. That is the first time that I did not see or catch any swarms during that following Summer. Even if the hobby beekeeper does not desire to increase their hive count with splits, there is plenty of interest among new beekeepers to purchase nuc hives. Or the beekeeper could marry the queenless part of a split to another weaker hive in the apiary.

The swarm gathers around the queen in or on a bucket.
Capturing a Swarm
The capture of a swarm may be as simple as shaking them into a hive from a hanging branch or have the complexity of extracting them from an enclosed building cavity. When a queen leaves a hive with a swarm she usually will land within 100 feet or so of the originating hive. Most of my early swarm catches have been within several 100 feet of my own hives. This might be an indication that they were coming from my hives. Although six large swarms within about two weeks coming from the only two hives I had one Summer seems a bit implausible.
Bees surround the queen to keep her warm, dry and safe until scouts find a new location for their new home. This could be any sort of building or tree hollow that the scouts agree upon. Although a swarm can be intimidating in appearance, they have engorged themselves on honey before leaving the hive and have no eggs, brood or stores to protect. As a result the swarm is relatively unlikely to sting and can be quite docile. The one reason bees tend to sting is if some are getting squeezed or feel threatened. The swarm may move off in a matter of hours or stay in the initial swarm location for several days depending on weather and how soon the scouts find a new home. So it is wise to capture a swarm as soon as possible to avoid their eminent departure.
The steps to capture a swarm are relatively easy. It is advisable to wear a bee suit and veil as the disturbance of the swarm may be enough to make them feel threatened. You will need some sort of container for transport, if it is not convenient to drop them directly into a new hive. A five gallon bucket will work well, particularly if it has a ventilation screen on the top or a cardboard box with ventilation screened holes in a few spots works equally as well. Once the container is under the bee swarm, a quick shake will drop nearly all into the container. I have seen a five gallon bucket fastened on the bottom of a ten foot pole that was used to reach a swarm a bit higher in a tree. Shaking the branch caused most bees to drop into the bucket. If they are on a non-shakable surface a bee brush may be necessary to gently sweep them into the container. Some beekeepers like to spread a bedsheet or tarp under the spot where the bees will drop to better contain those falling outside the container. If the queen fell into the container, most of the remaining bees will join her. If she has not gotten into the container, the bees will return to her and reform a new cluster around her location following her queen pheromones. You will need to give them a little time to resettle down after which the process can be attempted again. If able, leave the container sit until nightfall or dusk to give the remaining bees time to congregate in and around the container. After dark, the bees will have settled down and the ventilated container can be closed and taken to the new hive location. Within a day, they should be moved into their new hive. If you are not interested in capturing a swarm there are eager beekeepers that can likely be contacted through local clubs, government Ag or environmental agencies who will come to get the bees. Feral swarms are most desirable because they are genetically suited to the local area and tend to be less disease prone than those raised in the hundreds by commercial apiaries which are shipped as packages to beekeepers.

A ten frame deep on a barn mounted platform used as a swarm trap just prior to strap down.

A 40 liter swarm trap mounted on a barn stand just prior to strap down.
Swarm Traps
I have made it a practice to set out a swarm trap or two early in each season of beekeeping. I have attached two hive stands to the rear of a hip roof barn at about eight feet off the ground. A bit higher would be nice but that is the height of an inner beam that made a good attachment point. Nearly every Summer, I have had a swarm move into one of these traps. On occasion, I have simply set a single ten frame deep hive on a stand and occasionally a swarm moves into it. I think the empty drawn comb frames inside are a good draw for the swarm. My homemade swarm traps hold five deep frames with another five or six inch open space below the frames. Research has shown that 40 liters of space seems to be the ideal to draw in a swarm. The open space below, along with the five frames, works out to almost exactly 40 liters. In addition to the five drawn comb frames, I will add a plastic perforated baggy at the bottom with an inner paper towel that had some lemongrass essential oil drops added to it.
The plastic baggy is perforated in a dozen spots by poking a sewing needle through the baggy. Only a few drops are necessary and this seems to attract bees that are in a swarm state. My most unusual swarm catch occurred one Summer when I failed to remove an empty hive to storage. Sitting in the middle of my row of hives I had left it there for the other bees to clean out, a small bit of the remaining honey still inside. Every so often I checked it to see that no hive beetles or wax moths had moved in, but always failed to move it to storage. One day, I noticed bees carrying pollen into the hive which I thought a bit unusual for an empty hive. Come to find out, the bees coming and going, which I thought were just gathering the remaining honey, were actually a swarm that had moved in with a new queen that was laying a nice pattern of eggs and already had larva in surrounding comb. Nothing seemed to be missing from any of my other hives. That hive is now coming into its third year and still seems strong.
So, by managing your strong overwintered hives with splits and observance, you may be able to avoid losing much of your bee population to swarms. Use an increase in hive space and/or splits as a swarm management tool to increase your hive count and/or nuc resources for personal use or sell-able nucs. Your swarm experience could vary based on your conditions, environment or state of your overwintered hives.
]]>Click Here if you listened. We’re trying to gauge interest so only one question is required; however, there is a spot for feedback!
Read along below!
Found in Translation
Bees have an increasing say in soybeans
By: Jay Evans, USDA Beltsville Bee Lab
Farmers and scientists debate the extent to which one of our country’s favored crops, the soybean, benefits from honey bee visits. Nor are they sure that having bees visit soybean crops is a net positive for the bees. Despite research documenting strong benefits to soybeans from honey bee visits (dating since the youth of former Bee Culture editor Kim Flottum, https://www.beeculture.com/found-in-translation-19/), a perusal of thousands of studies related to soybean farming shows little emphasis on how and when bees should be deployed. As one metric, a March 2023, Google Scholar search of papers mentioning “soybean yield” and “honey bee” provided 276 references. The same search excluding the term “honey bee” provided 62,200 references. This overall trend has not improved in recent years; papers mentioning soybean yields that do not mention honey bees number 5,110 since 2022, while only 32 papers mention honey bees. Fortunately, those 32 papers provide some really important advances. The upshot is that bees can greatly improve soy production, while potentially gathering a resource for themselves and their keepers. What remains to work out:
- How can beekeepers practice safe soy?
- How can growers choose varieties and management practices that harness bee visits to boost production of a vital row crop?
- How can the two sides meet up to work out deals that benefit both industries and the environment?
 On the soy side, honey bee pollination impacts were described this month in a freely available paper from Decio Gazzoni and João Paz Barateiro (Gazzoni, D.L. & João Vitor Ganem Rillo Paz Barateiro. 2023. Soybean yield is increased through complementary pollination by honey bees, Journal of Apicultural Research, DOI:
On the soy side, honey bee pollination impacts were described this month in a freely available paper from Decio Gazzoni and João Paz Barateiro (Gazzoni, D.L. & João Vitor Ganem Rillo Paz Barateiro. 2023. Soybean yield is increased through complementary pollination by honey bees, Journal of Apicultural Research, DOI:
10.1080/00218839.2022.2161219). These authors showed that, with the right conditions and soybean varieties, honey bees increased soybean yields in controlled environments by 8.5-18.2% in four trials across three years. This increase is not as dramatic as other studies from different cultivars, but still reflects a lot of beans. Hannah Levenson and colleagues at North Carolina State University also showed recently that supporting bees merely by expanding local non-crop habitat led to a significant difference in soybean seed (bean) weights. In an exhaustive survey of 7,000 bees in the field, they found that 30+ bee species had collected soybean pollen but honey bees tended to be more faithful than others for soy versus alternatives (Levenson, H. K., A. E. Sharp, and D. R. Tarpy. 2022. Evaluating the impact of increased pollinator habitat on bee visitation and yield metrics in soybean crops. Agriculture, Ecosystems & Environment 331:107901, https://www.sciencedirect.com/science/article/abs/pii/S0167880922000500).
If bees are generally good for soybeans, are these visits doing bees any good? Chia-Hua Lin and colleagues at The Ohio State University have been on that story for some time and recently published a complex study asking whether bees 1) make it to abundant local soybean fields and 2) bring home resources for their colonies (Lin, C.-H., Suresh, S., Matcham, E., Monagan, P., Curtis, H., Richardson, R. T., & Johnson, R. M. 2022. Soybean is a Common Nectar Source for Honey Bees (Hymenoptera: Apidae) in a Midwestern Agricultural Landscape. Journal of Economic Entomology, 115(6), 1846-1851. doi:10.1093/jee/toac140). In a citizen-science twist, the scientists asked members of the Ohio State Beekeepers Association to bring honey collected by bee colonies from across the state to their Fall meeting. This honey was screened for the presence of different pollen types under microscopy. As indicated by the title, soybean pollen was commonly found in Ohio honeys. More than half of the screened honeys held soybean pollen, and this increased for honey derived from foraging in July and August, when soybean flowers were most common. Finally, the authors used the waggle dance, the signal bees use within their colonies to direct nestmates to good foods, to show that returning bees are eager to tell their nestmates about soybean rewards. For medium-distance flights, returning bees were more likely to ‘dance’ that they had visited soybean fields than other fields, complementing the pollen collection data and saying that bees preferentially target soybean fields over the alternatives. Dr. Lin has backed up this work with some truly remarkable studies covering the attractiveness of dozens of soybean cultivars to bees in common gardens (e.g., https://ohiocroptest.cfaes.osu.edu/soy2022/2022_OSPT_pollinator_report.pdf) and is working relentlessly to improve cross-pollination between beekeepers and soybean growers.

Team B & B (Bees and Beans) collecting flowers in soybean plots last Summer. The white stakes are Karlan Forrester’s audio recorders. Photo provided by Chia-Hua Lin from the Rothenbuhler Honey Bee Lab at The Ohio State University
In ongoing work, graduate student Karlan Forrester (working with Chia-Hua Lin and Reed Johnson at Ohio State), has worked out innovative methods for tracking bees as they zero in on soybean flowers, while also confirming that certain soybean varieties are more rewarding, and hence attractive, to discerning bees (Forrester, K. C., Lin, C.-H., & Johnson, R. M. 2022. Measuring factors affecting honey bee attraction to soybeans using bioacoustics monitoring. BioRxiv, 2022.2011.2004.512777. doi:10.1101/2022.11.04.512777).
In looking for soy-bee stories that describe ways to enhance this partnership, I came across a series of fascinating works from the other side of the world. Dr. Dolapo Bola Adelabu, a researcher from the Free State of South Africa, and his colleague Angelinus Franke, found remarkable increases in soybean yields that can be attributed to visits by bees and other pollinators (Adelabu, D.B., Franke, A.C. 2023. Beneficial Role of Pollination and Soil Fertility for Soybean Production in Mountainous Farming Conditions. In: Membretti, A., Taylor, S.J., Delves, J.L. (eds) Sustainable Futures in Southern Africa’s Mountains. Sustainable Development Goals Series. Springer, Cham. https://doi.org/10.1007/978-3-031-15773-8_5). These yields were greater than 50% when combined with optimal fertilizer supplementation of crops (Nitrogen and Phosphorous), with less striking increases under poor soils. Farming in this region of southern Africa, in a rugged corner of the Free State, is distinguished by “smallholder” farms, where farms are interspersed with homes and natural areas. This farming scheme allows for both wild bee habitat (honey bees are not routinely kept in hives here) and presumably a range of alternate food sources for bees when soybeans are not in flower. In conversing with Dr. Adelabu, the studies did not distinguish Apis mellifera from other bee species, but it seems likely that honey bees were a major member of the pollinating community. Thanks to this research, the services bees provide in terms of local soybean yields, among other crops, justifies the work needed to keep healthy bee habitat. The two scientists in this work are also more broadly interested in schemes to provide healthy nutrition to a fairly dense human population, while maintaining a sustainable environment, ( e.g., https://www.ufs.ac.za/aru/aru-team/aru-team/prof-angelinus-franke). Hannah Levenson phrases it well in her article, “As such, pollinator habitat should be designed to provide resources across the entire active season to help these important pollinator populations, especially since many crops have short bloom durations.”
One hope from all this research for the U.S. will be improved dialogue between beekeepers and soybean farmers, ideally driven by profits on both sides. This dialogue will help bees collect soy flower resources while minimizing collateral damage from agricultural practices, including the need to treat for crop diseases and insect pests. In the meantime, what are the best practices for beekeepers around soybean farms? The Honey Bee Health Coalition has focused on this issue, leading to a draft of guidelines led by Adam Dolezal at the University of Illinois showing how management practices, from pesticide applications to habitat, can be more bee-friendly (https://honeybeehealthcoalition.org/resources/soybean-best-management-practices/). Making more food on fewer acres is good for the planet and the economy, and it is great that scientists and farmers on both sides are tackling the soy-bee system in a rigorous way.
]]>By: Nina Bagley

Mrs. Helen Goodsell Acklin

Ethel Acklin Calvert, circa 1929
Breezing through my 1900s Gleanings in Bee Culture Magazines, I found a beekeeping woman and her daughter who caught my interest: Mrs. Helen Goodsell Acklin and her daughter Miss Ethel Acklin from St. Paul, Minnesota. Ethel would marry Howard Root Calvert, son of Maude Root Calvert, who was the daughter of A. I. Root.
Helen Goodsell was born in New York on May 3, 1857. Her parents, Jessie and Laura Goodsell, were both born in New York. Helen was the youngest of five children. Her parents moved to Wisconsin when she was very young. She attended a country school and then a village school, preparing her for a career in teaching. Her passion for honey bees started at a young age. In her teens, she told herself that she would have bees someday, but it would be a while before this happened.

Helen’s Divorce Paper, Dec. 8, 1885
At fifteen, Helen married her first husband, Phillip P. Jewell on November 11, 1872. He was ten years older than Helen. I couldn’t find much information about Phillip other than he was in the Civil War and that Helen served him divorce papers. In 1885 you needed proof and a reason for getting divorced. The grounds for divorce were adultery, desertion and abuse. The ink had faded on her divorce papers and was hard to make out. I’m sure she had her reasons- the Civil War was brutal and many men suffered long term mental and physical illnesses from serving in it.
 Phillip P. Jewell didn’t show up for court. Helen was granted her divorce on December 8, 1885 at the State of Wisconsin Circuit Court for Polk County. While living in St. Paul, Minnesota, in 1884, she met her second husband. James C. Acklin, who was working as a contractor. He had been married before and had a daughter, Annie. The two married on December 8, 1885. The same day Helen was granted her divorce. She was 28 years old. What a courageous young woman! Talk about killing two birds with one stone.
Phillip P. Jewell didn’t show up for court. Helen was granted her divorce on December 8, 1885 at the State of Wisconsin Circuit Court for Polk County. While living in St. Paul, Minnesota, in 1884, she met her second husband. James C. Acklin, who was working as a contractor. He had been married before and had a daughter, Annie. The two married on December 8, 1885. The same day Helen was granted her divorce. She was 28 years old. What a courageous young woman! Talk about killing two birds with one stone.
James C. Acklin was born in Pennsylvania in 1857. He was a contractor and builder, and was a prosperous man. A. I. Root described him as dignified, gentlemanly, quiet and significantly large in stature. The time for bees finally came for Helen shortly after they were married. Fortunately, some bees were on the lot where they were building a home and thus began their beekeeping journey. For a present to his wife, Mr. Acklin, made her seventeen colonies of American hives. The bees were not the most friendly, so they replaced them with a gentler stock. The frames were all glued together and made a mess out of the hives. It took some doing to pull them apart.

J.C Acklin
July 1, 1906 The Western Edition

Helen G. Acklin
Gleanings Eastern edition February 15,1906
Nevertheless, Mr. Acklin meant well, and Mrs. Acklin transferred them to Langstroth hives with the assistance of her husband. In 1890, they went west to California, but in the Fall of 1891, they returned to St. Paul, where they planned on building a life together. Their daughter Ethel was born in the Winter of 1892. And the following Spring, 1893, they began handling beekeeping supplies. Very little building was going on during the hard times, and Mr. Acklin secured a position as a lumber inspector with the Great Northern Railway. He worked for several years. Both of them tried their best to prevail. Mrs. Acklin kept a watchful eye on their daughter and ran the bee supply business. Like any good business, with a vision and hard work, it will grow over time, and this is what happened. The work became overwhelming! Her husband effectively resigned from his position with the railroad to help his wife out full-time with the bee supply business. He devoted his time to his family and the business of bees. Mr. and Mrs. Acklin both were active in the Minnesota Beekeepers Association. They both were closely associated with helping each other and working side by side until Mr. Acklin’s sudden death in 1906.
After the death of her husband, Mrs. Acklin continued with beekeeping, having her ups and downs working her bees. She had over 100 hives and several out-apiaries. Even then, disaster sometimes comes knocking on one’s door. One morning she realized water overflowed the cellar apiary, and sand was running into the entrances and drowning the bees. I’m sure if you kept bees long enough, you would have had issues with your bees. But can you imagine the cellar filling up with water? The colonies floated around like fishing bobbers until rescued by men with rubber boots and a long pole!

Ethel Helen Acklin, 13
1913
A woman with less love for the honey bee and less perseverance would have surrendered in despair, and to add to it, she felt alone. But Mrs. Acklin kept on, learning something by hard knocks, and from experience and her bee books, she followed Doolittle’s method of raising queens. It would be discouraging and make one choose another occupation, but with her guiding principles, she prevailed. With her small beginnings, Mrs. Acklin was one of the most successful beekeepers in St. Paul, Minnesota. And she was well known in Wisconsin too. Running a sizeable queen-rearing apiary at her home, she was a prominent dealer in beekeeping supplies for over fifteen years, alongside being an attentive mother.

“MISS ETHEL ACKLIN AND HER BEE-KEEPING SONGS AT THE CHICAGO CONVENTION.”
Her daughter Ethel knew more about bees than most young women and men. She accompanied her mother and father to the bee conventions. Ethel remembers in 1900 when she was nine, singing solo and chorus songs, the music by Dr. C.C Miller, during the National Convention in Chicago. How she enjoyed the time with her parents. The death of her father was not easy on both mother and daughter.
Mrs. Acklin continued her business for a few more years, but her failing health made her make a change of her occupation and climate. So she moved back to California in 1908, eighteen years since she had last been there. She didn’t entirely give up on bees. At fifty-one, Mrs. Acklin, with her daughter, bought a home in Glendora, California, and purchased an orange grove twenty-five miles outside of Los Angeles. She became a rancher and an orange grocer keeping a few hives.
She also attended the bee conventions. She edited the Beekeeping in Southern California department in Gleanings. Mrs. Acklin was against beekeepers that sold adulterated honey and worked aggressively to put them out of business! She wrote in Gleanings in Bee Culture: “I wonder how many beekeepers know that honey adulteration is happening right in our midst; if beekeepers work together, these swindlers can be put out of business. Whether it be the small grocer who puts just a little glucose in the honey to keep it liquid or the wholesale man who mixes tons, the effect is the same. People soon take a dislike to glucose honey. They stop eating honey altogether. So beekeepers lose money on two counts, less honey being consumed while the output increases. How can this adulteration be stopped? All beekeeper’s associations in our State, whether county clubs, district unions or State organizations, should unite under one banner in fighting this evil, and send a large and enthusiastic delegation, composed of delegates from each society, to the legislature this Winter to represent the beekeeping industry of our State.” Gleanings, 1910 (pg. 749).
When A. I. Root Co. established a branch office in San Francisco, Mrs. Acklin was given charge of it and continued to work until her death on May 30, 1915. A. I. Root enjoyed visiting Mrs. Acklin’s home in Oakland when he was on business in California. And found her to be commendable, a good businesswoman and a loving mother.

Howard Root Calvert
1923
A. I. Root’s grandson Howard Root Calvert took care of the A. I. Root Company’s exhibit booths at the San Francisco expositions. And it was during one of these visits Howard would meet Miss Ethel Acklin. They announced their engagement to be married. Ethel’s mother passed away a few months before the wedding. The two were married on Tuesday, July 6, 1915, at the home of the groom’s parents, Mr. and Mrs. J. T. Calvert, in Medina, Ohio. Ethel was grieving her mother, her last surviving relative, and is said to have held up bravely. The death of the bride’s mother made it necessary for Ethel to be married in Medina, Ohio.

Mr. and Mrs. Howard Root Calvert
Immediately following the ceremony, the couple drove to Elyria, Ohio where they started their long trip back to California. Mr. and Mrs. Calvert were both in charge of the San Francisco office, taking hold where Ethel’s mother left off. Mr. and Mrs. Calvert dealt with life’s realities and an active bee business. Ethel was said to have been “the bee man” of the establishment. In 1916, Mr. and Mrs. Calvert returned to live in Medina, Ohio, where they would start their family. Howard continued working for his family’s business, the A. I. Root Company.
The family had ultimate happiness and then ultimate loss. Mrs. Calvert’s husband died suddenly as a young father at thirty-two in a tragic plane crash while giving lessons on June 27, 1924, in Akron, Ohio. He was not flying the plane but the student, Mrs. Whichershelm, was. What a tragedy! Now widowed at a young age, Ethel had three young daughters to think about. Rebecca, seven; Roberta, five; and Ruth, four. Her daughters were all she had left, falling back on her husband Howard’s strength, her mother’s tenacity, and her own will and faith, Ethel would return to California and overcome by providing for her girls, owning her own home and bee supplies company just like her mother had done.

Alfred Francis Nippell yearbook picture
It would be thirty-six years before Ethel would remarry. But I always say, “there’s a lid for every pot.” In 1960, she married Mr. Alfred Francis Nippell. He was fifty years old, his occupation was a bookkeeper, and he had never been married; Ethel was sixty-seven. They were married for twenty-eight years. Ethel slowed down regarding the bees, just keeping some bees in the country. Ethel was ninety-five when she passed away with her family by her side on January 8, 1988.
She shared the love of the honey bee. Endured the loss of her parents and her husband at a young age. She raised three daughters who were related to the Root family. She had four grandchildren and eleven great-grandchildren, which gave her much joy. Her second husband Alfred provided her with love, companionship and security. He passed away at the age of ninety in 2000 in San Diego, California. Mother and daughter both having parallel lives. “I admired their tenacity, entrepreneurship and strength; the busy bee has no time for sorrow.” —William Blake
Nina Bagley
Ohio Queen Bee
Columbus, Ohio

Photo 1. Amy Vu portrait. Photo Credit: Randy Fernandez, UF/IFAS Entomology and Nematology
From the University of Florida Honey Bee Research and Extension Laboratory – Part 4
By: Amy Vu
Hi everyone, my name is Amy Vu and I work at the University of Florida’s Institute of Food and Agricultural Sciences Honey Bee Research and Extension Laboratory (UF/IFAS HBREL). Many of you may be familiar with my voice in a podcast called “Two Bees in a Podcast” (if not, now is the time to get your smart phones out and search for it!). This is the first time I have written for Bee Culture and I am excited for the opportunity. I have been with the University of Florida since 2016, beginning my career as a county Extension agent in Orange County (Orlando and the surrounding area). Since then, I transitioned to the HBREL in 2019 and the rest is history. My current title at UF/IFAS is “State Specialized Program Extension Agent, Apiculture”, and I somehow still fumble over the words in my title anytime I introduce myself. To sum up what I do: my work involves working with beekeepers and their stakeholders, identifying needs, conducting activities and evaluating programs. I will elaborate more throughout this article. If you have been following the past few months of this series, you have learned that there are three key components to a Land Grant Institution (LGU) (Instruction, Research and Extension). Jamie discussed the overview of LGUs and research. Cameron discussed formal instruction program. In this article, I have the honor of writing about the third leg of the stool (and my personal favorite) to bring it all together, Extension! As Jamie mentioned, all universities engage in teaching degree-seeking student and research, but not all universities provide one of the LGUs critical missions, Extension. One thing you will notice throughout the article is that Extension cannot happen without connections and collaborations with our stakeholders and that feedback is extremely important. I am here to share the good, the bad and the ugly. Next month, our program coordinator, Louis, will be sharing with you about the Extension workshops we conduct at our lab.
Before I continue further, I would like to share a bit about myself. I was born and raised in Overland Park, Kansas (Go Chiefs!), a city kid with no agriculture exposure. It was not until a study abroad program in Ecuador in 2009 that my attention turned to agriculture and where our food originates. I immediately fell in love with understanding our world’s food systems and completed my bachelor’s degree at Kansas State University in Agronomy with an emphasis on Soils and Environmental Science. From there, I moved to Blacksburg, Virginia, where I worked on a Master’s degree in Agricultural Leadership and Community Education at Virginia Tech. In 2014, a group of colleagues in graduate school and I decided that we wanted to try a new endeavor: beekeeping, because… why not? It seemed like a fun new challenge. Little did I know, that experience would change the course of my entire career. After graduation, I decided to move to Florida, since I am a scuba cave diver and wanted to be closer to the freshwater springs (did you know that Florida has one of the largest concentrations of freshwater springs in the world?). The springs brought me to Florida, but the bees have kept me here. During my time working in Orange County, I attended the local Orange Blossom Beekeepers Association (OBBA) monthly meetings. After a few meetings, I started to realize that the association was spending ¾ of the meeting time answering basic honey bee terminology and having to take a step back each month to teach newbies equipment terminology. They were losing more advanced beekeepers, and I wanted to help, and knew that I could with my Extension programs. The association and I started working together, which resulted in a beginner beekeeping series. The association helped me promote it and we timed it perfectly, so my programs would be held the Tuesday following monthly meetings. This helped reduce the beginner beekeeping time at the monthly meetings and allowed the association to focus on more advanced topics.

Photo 2. Amy cave diving in Madison Blue Spring State Park. Photo credit: Lauren Wilson
What is Extension and an Extension Agent anyway?
Something I hear often is “So… is your job to run the UF honey bee lab’s social media page?” This is something I have heard before, and the answer is “yes, kind of, but also… no, I do so much more than that.” Social media is only one method of communication for how we connect with stakeholders and market our programs. Just to recap and quote Jamie’s first article: “Faculty at LGUs engage in formal education (teaching students) and informal education (teaching everyone else). The latter is called Extension.” Let’s start with the basics. We define an “agent” as a person who acts on behalf of another person or group. In Extension, my job is to bridge the gap between industry, researchers, government agencies and more by providing non-formal education and learning activities to all types of individuals. In short, we are a resource for our stakeholders. Extension originated to help mostly with rural areas but has evolved to work with both rural and urban areas. We are messengers and disseminate science to our stakeholders.
It is always extremely difficult for me to explain to people what my job is and how it relates to UF/IFAS, but in a nutshell, my job is to share research and do my best to stay up to date with new and emerging topics. In Extension, there are many programmatic areas. The most common programs and topics that are well known are Horticulture, Agriculture and Natural Resources (ANR), 4-H, Family and Consumer Services (FCS) agents.
The History of Extension
The Smith Lever Act formalized Extension in 1914. At the time, Extension services were provided exclusively to rural agricultural issues. Over 50% of the U.S. lived in rural areas, and 30% of the workforce was engaged in farming. Because of Extension, farmers increased their productivity, allowing farmers to make better management decisions. This resulted in a higher production of food. Basically, there was research being conducted at the university, but the practical research was not being shared with farmers. Extension agents were hired to bridge the gap, hold workshops to help growers, and act on behalf of growers to let researchers know what practical research could be conducted to help the industry. Extension has changed immensely in the last century. Today, less than 2% of Americans farm for a living, and only 17% live in rural areas. That said, Extension specialists must be creative with their programs, increasing their audience size by diversifying their workshops, activities and communication. Programs in urban areas have increased and the specialties have expanded beyond farming and canning classes. In the United States, Extension/Ag offices are located in over 3,000 counties.

Photo 3. Picking up our first packages in 2014. Pictured here: Amy Vu, Rachel Kennedy, Sarah Halvorson-Fried and Becca Ligrani
What is an Extension Program and How are They Conducted?
Extension is a federal, state and county partnership. The purpose of Extension is to bring the most recent and cutting-edge research from laboratories to those who can use the information to learn and implement skills into practice. This is done through Extension programs. There is a theory to what we do as Extension agents and how we develop programs. Developing a program requires planning, implementing, evaluating and doing that over and over (and over) again. Agents will identify an overarching theme (for example, collaborating with beekeepers to increase honey bee colony health), and then plan workshops or create materials that fall in line with the overarching theme (for example, holding a hands-on honey bee workshop focusing on honey bee stressors, like Varroa management). These themes are called “Programs” (with a big P!), which is contrary to what people may think a “program” is. Most individuals hear of the word “program” and think of a workshop or one hour talk on honey bees. In Extension, when we hear the word “Program”, we think about the big picture theme of what we are trying to accomplish. To develop, implement and evaluate our programs, we use something called a Logic Model (and I know that as soon as I mentioned the words “logic model”, I hear every Extension Agent’s eye roll and grunt from here). All joking and sarcasm aside, a Logic Model is an extremely helpful tool that agents use to plan an Extension program. It is a blueprint and vision for our programs to help us focus on the bigger picture.
Using a Logic Model
It always starts with a needs assessment and identifying the situation
Conducting a needs assessment is the foundation of a good Extension program. Understanding the needs of a community is crucial to know how to move forward and how to disseminate information. Understanding your audience helps with being successful. This can be done by simply asking questions and listening. Extension is all about the relationships we build with our stakeholders. Without the collaboration, a successful program cannot exist. As apiary educators, we are constantly on the search to hear what is happening in the field. Are we hearing about high colony losses? Why do beekeepers believe they are losing colonies? What time of year is it? Has this happened before? Are we concerned about a new invasive pest or disease?
Let’s look at a situational example specific to Florida. This example can be scaled up to be used all over the world: There are over 5,000 registered beekeepers managing approximately 700,000 colonies in Florida. Of these 5,000 beekeepers, hobby (zero to 40 colonies) and sideline (41-99 colonies) beekeepers make up ~92% of all beekeepers, while the rest (~8%) are commercial beekeepers (100+ colonies) (Florida Department of Agriculture and Consumer Services – Division of Plant Industry (FDACS DPI), personal communication). Beekeepers report an annual loss of 40% colony decline. The reasons identified for the losses are Varroa, nutrition, queen quality and pesticides. Thus, Extension programming focused on how to monitor, manage and control honey bee stressors is necessary to minimize colony losses.
In this scenario, here are the Programs (overarching themes):
- Honey bee health: best management practices are needed related to Varroa, nutrition, queen quality, minimizing pesticide exposures, etc.
- Training the trainers: The need is to work with UF/IFAS Extension Agents, apiary inspectors and other Extension educators to extend this content.
How do we acknowledge the situation?
Target Audience
First, we must consider who the program is targeting. Who will show up and in what ways will they show up? Is an e-mail listserv better than an in-person workshop? What good is a program if you hold a workshop and no one attends? Typically, our target audiences are beekeepers: backyard, sideline and commercial. But it is important to recognize that not all beekeeper’s needs are the same. Other audiences’ agents work with and include non-beekeepers to teach them about the importance of honey bees to our agriculture.
After identifying needs and our audience, we must analyze the needs and turn them into objectives. It is typical for us to have two to three objectives per Program. We use SMART objectives to hold ourselves accountable. So what does SMART as an acronym mean?
Specific: Who is the audience and what is the goal? Are we addressing a specific action or is it too complex or broad?
Measurable: The results should be quantifiable—the goal will have a target timeline or benchmarks designed to measure progress. How will we know when the objective has been accomplished?
Attainable: Is it realistic? Are there clear steps to adopt any recommendations?
Relevant: Does the objective align with the mission and vision of the organization?
Time-bound: It must have a beginning and pre-determined end. Also, there should be timed milestones at which progress can be evaluated along the way. Developing a timeline helps one evaluate performance and efforts on a regular basis.
So here is a SMART objective that I will continue to refer to throughout the article:
“At least 75% of individuals who participate in the UF/IFAS HBREL’s events will begin monitoring for Varroa using an alcohol wash or sugar shake within six months of attending a program. This will be measured by post program evaluations and follow-up surveys.”
Okay, so now that we have an objective, let’s talk about the fun part – the activities!

Photo 4. A group of UF/IFAS Extension Agents in an in-service training learning about Varroa monitoring using the alcohol wash method.
Educational Methods and Activities
Inputs – what we invest
Extension’s job is to translate science-based research into digestible bits—whether that be written, verbal, in person or virtual. What money, facilities, technology, people and other resources are needed? What is the time commitment? What materials do we need to conduct a workshop? Can we conduct a Varroa monitoring class without the proper equipment? Do we have the appropriate classroom space or apiary space to conduct the program? What personnel (staff, volunteers, etc.) are needed to implement the workshops? Do we need supplementary materials to support our activities (like PowerPoint presentation, fact sheets, a brochure, etc.)? What about technology? When COVID began, we had to be creative and start using Zoom. Our inputs changed and we needed access to Zoom and other programs to implement our trainings. Knowing what inputs are necessary are important to think about to ensure the success of the program.
Outputs
Activities – what we do
The most common activities for apiculture educators are in-person workshops in a classroom or out in the apiary. Sometimes we are invited speakers at beekeeping association meetings. Sometimes we plan and hold workshops in person or a talk on Zoom. This is the piece of Extension that is the most visible. The activities we conduct are completely dependent on our target audience. For example, sometimes it is easier for hobbyist beekeepers to attend an evening or weekend event, so we will hold larger classes on hobbyist topics during that time. Other times, commercial beekeepers may be working and unavailable, so it may be easiest to conduct a single site visit to their operation. Another activity that is extremely common is answering beekeeping related questions from our stakeholders, whether that be over the phone, e-mail or a message on social media. It is important that we are available as a resource.

Photo 5. Two Bees in a Podcast logo
Here at the University of Florida, we have key activities with which you may be familiar. Our Extension Coordinator, Louis will elaborate on these activities in next month’s article: the UF/IFAS Master Beekeeper Program, BeeLearning Short Courses, UF/IFAS Bee Colleges, Two Bees in a Podcast and the UF/IFAS Honey Judge Program. Additionally, the UF/IFAS honey bee team regularly provides lectures and workshops at various training events.
Outcomes
What do we want to see accomplished in the short, medium and long term from our programs? How do we identify these outcomes? Let’s revisit our example objective mentioned earlier: At least 75% of individuals who participate in the UF/IFAS HBREL’s events will begin monitoring for Varroa using an alcohol wash or sugar shake within six months of attending a program. This will be measured by post program evaluations and follow-up surveys.
Short term outcomes focus on gaining knowledge, raising awareness, creating a positive attitude, developing a skill/opinion or increasing motivation. From our example, a short-term outcome could be that participants increase their knowledge about Varroa monitoring and learn how to collect a sample using an alcohol wash. Developing this skill has increased their motivation and attitude to conduct samples more frequently.
Medium term outcomes focus on seeing a behavior or practice in motion. From our example, a medium-term outcome could be that six months after the training, beekeepers have started monitoring for Varroa monthly using an alcohol wash and make informed decisions on whether to treat for Varroa or not, based on thresholds.
Long term outcomes are the ultimate impact. These are usually social, economic or environmental. When making informed decisions related to Varroa management, long term outcomes involve minimizing overall colony losses, which can increase production, and in turn increase profitability.
How do we know our programs are effective?
I will not bore you with the 30-page report we have to submit annually, but I will describe some ways we report our programs. After conducting a needs assessment, coming up with objectives and holding an activity/workshop, we must show that our programs have made a difference. For formal Instruction, students take exams, write papers and end the semester with a grade. Researchers are evaluated by the number of grants they bring in and how many peer-reviewed publications are accepted each year. For Extension, we must show that we are changing lives for the better… CHANGING LIVES! Easy to measure, right? Our big question is: “so you’ve put all this work into a program… SO WHAT?” That’s probably a bit harsher than reality, but you get the gist. If you have taken part in an Extension activity, you have likely taken a pre- and post-test, filled out a survey immediately after a program and have been asked to fill out a follow-up end of year survey. We also use general feedback, verbal and written, in consideration for our evaluation. Evaluation is the most critical component of Extension. It determines whether we move our programs forward (or not). I cannot stress the importance of evaluation enough. In short, we must show that our Extension efforts are making a positive difference, and if they are not, maybe it is time to rethink how we conduct our programs.
International Extension Activity Highlight
Our Extension efforts extend well beyond our county, state and country. I would like to highlight one international activity in which I participated and maybe you can identify some of the pieces of the logic model I mentioned earlier. I was fortunate to be involved in the United States Agency for International Development (USAID)’s, Partners of the Americas, Farmer-to-Farmer program in February 2023. As a volunteer in the Dominican Republic, I was joined by a Florida commercial beekeeper, Chris, and his wife Melissa Vasquez, with Heritage Bee Farm, LLC. We had the most wonderful field officers, Maria Montas and Francisco Mendez, who helped translate technical honey bee content in Spanish. We worked with ASAJA (their beekeeper association) in the beautiful Jarabacoa mountains for 15 days. The beekeepers were all in their first five years of beekeeping. Many of them were beginner beekeepers, while a few members of the association were not. Those few aspired to become full time beekeepers and wanted to learn more about general management and queen production. It was a huge breath of fresh air (I did not know what to expect) to see that the honey bees were strong and had all the pollen and nectar available to them (the beekeepers do not need to feed there, since there is so much pollen and nectar flow). The beekeepers also do not have small hive beetles present on the island (lucky them!). During our initial meeting, Chris and I learned that the beekeepers were interested in learning more about pest and disease management, equipment management and queen production. We saw Varroa on the bees, thus prompting me to ask the beekeepers what they used (and how often) for treatment. They use thymol and oxalic acid-based products. Prior to our visit, beekeepers were only monitoring and treating once a year. In our program, we spent every day conducting a needs assessment and evaluating colonies, implementing short hands-on workshops and providing recommendations on when to graft larvae for queen production. We also piloted the use of coconut water as a media for queen production and discussed how often to monitor for Varroa, when to treat and the importance of rotating active ingredients. At the end of the two weeks, we provided resources for the beekeepers to help continue with their education. On the last day, Chris and I delivered a presentation about what we learned, what we observed, and provided recommendations (using SMART objectives) to the association on how they could be successful as a team in the long term. I evaluated the program by interviewing 10 program participants, asking about potential challenges, what they learned and what other resources or training they still need to be successful as a beekeeper. I hope to spend the next few months evaluating the data. I am fully convinced that our short time in the Dominican Republic will have long term outcomes for the beekeepers and it was an extremely rewarding Extension experience.

Photo 6. Amy speaking to media to discuss the importance of honey bees and commercial beekeepers to agriculture after Hurricane Ian left devastating losses to Florida beekeepers in 2022. Photo Credit: Bori Bennett
What else do Extension agents do?
Okay, so back to Apiary Extension! We discussed needs assessments, developing programs, conducting site visits, speaking engagements to beekeeper associations, responding to phone calls and e-mails, creating content to support our programs and program evaluations. What else do agents do? You will find us at group meetings, speaking at professional conferences, writing and receiving grants, and being involved in other committees at our university and state associations. It may seem like we are everywhere, and that is because we are! Having the ability to be everywhere can be great, but it is all about balance.
Challenges of being in Extension
Not everything in Extension is a rainbow or unicorn. Being in Extension is the most rewarding career (in my humble opinion), but there are some struggles that many agents face. First, it is a blessing and a curse to have the ability of being creative with a program, which usually results in wanting to help and say “yes” to everything. There are so many potential projects, and simply not enough time to do them all. Focusing on long term projects when smaller “fires” arise and having to prioritize projects is crucial. When you speak to an Extension agent who has been successful in their career, the first thing they will tell you is to “focus on the big picture and learn how to say no.” Taking on every single project often results in major burnout and it can be very detrimental to our programs. Agents will feel like they are doing so much daily, but at the same time, not doing anything at all. This is something that is often seen, and the reason agents will leave their positions and transition to something else.

Photo 7. ASAJA Beekeepers Association in Jarabacoa, Dominican Republic. The participants were part of the USAID, Partners of the
Americas, Farmer-to-Farmer Program.
Second, agents are required to wear many hats. We are the event planner, marketing specialist, evaluation guru and content specialist. We must stay up to date with the times (whether that is jumping onto a new social media platform, creating content that is user friendly, etc.). We need to know how to design and use multi-media, be an expert in video, photo, or podcast production. We have administrative paperwork, we are expected to submit publications, speak at conferences and respond to thousands of e-mails and phone calls, and media requests at any moment.
Lastly, working with many types of personalities and people can be great, but also challenging. Anyone working with people has likely encountered a conflict before and knowing when and how to handle a conflict is crucial. It is important to remember that needs and priorities can differ from person to person. Facilitating conversations and relationships is important to get everyone on the same page to identify one common goal.
“The Good”
Despite some of the challenges an agent can face, I personally feel like I have the best job in the world. I will end this article with the positives of being in Extension. Extension agents have the privilege of working with people who become our family and friends. Many times, it becomes more than just a job, and it becomes the genuine connections we make with our stakeholders. I get to know personalities on an individual level. I meet their families, friends, pets, learn about their other hobbies, and so much more. I have watched beekeepers’ children grow up before my eyes. I have seen beekeepers progress from 50 colonies, to 100, and then to thousands of colonies. I consider beekeeper successes my successes.

Photo 8. Yancarlos Castillo grafting larvae to learn about honey bee queen production in Jarabacoa, Dominican Republic as part of a USAID, Partners of the Americas, Farmer-to-Farmer Program.
Second, I have seen groups of beekeepers work together to make a huge difference for the industry. Just one example is the building of our UF/IFAS HBREL facility. The $4.5 million facility came from the collaborative efforts of the Florida State Beekeepers Association, the Florida Department of Agriculture and Consumer Services, Florida beekeepers and other stakeholders. To watch a simple idea turn into a reality is incredible to see. “Never doubt that a small group of thoughtful, committed citizens can change the world; indeed, it’s the only thing that ever has.” —Margaret Mead.
Lastly, as Extension agents, we are fortunate to have the opportunity to be flexible with our programs and try new ideas. Extension agents will never have the same day at work. Some days are in the office, some days are in the field and others are presenting to local, state and national associations. We travel around the world to conferences or workshops meeting beekeepers and other individuals in the beekeeping industry, from regulatory/government personnel, non-profit organizations, researchers and other apiculture Extension specialists around the world. We are fortunate to network and work with the various stakeholders who play a piece in this large puzzle. The beekeeping community is so small and knowing what part everyone plays makes a huge difference.
In conclusion, being in Extension is not just a job, but a lifestyle. I love working in Extension and with beekeepers around the world. I enjoy connecting with beekeepers, hearing what is going on in their apiaries and being involved with the industry. I feel like I am making an immediate difference in the lives of beekeepers of all operations and hope to continue to serve beekeepers well into the future. Next month, our extension coordinator, Louis Dennin, will highlight specific activities we do at our lab. Thank you beekeepers, for all the great work you do, and allowing me to be part of the beekeeping community.
]]>