By: Gard W. Otis
The Yellow-legged Hornet, Vespa velutina, has been discovered in the state of Georgia! This social wasp, native to Asia, preys extensively on honey bees and other pollinators. Its arrival in North America, while not wholly unexpected, is a cause for alarm for beekeepers and agriculture in general. What is this wasp? Why is it a concern? What can we do to control it? And how concerned should we be at this time?

Figure 1. Yellow-legged Hornet, Vespa velutina, at rest. Photo taken in France, courtesy of Patrick Le Mao

Figure 1. A Yellow-legged Hornet hovers in front of a hive, awaiting incoming forager bees. Photos taken in France, courtesy of Quentin Rome / Muséum national d’Histoire naturelle, Paris, France.
V. velutina is a large wasp, approximately 0.7–1.0 inch (1.7–2.5 cm) in length and with distinctive yellow legs (Fig. 1). It is a social insect, with colonies composed of numerous workers and a slightly larger queen (Pérez-de-Heredia et al., 2017). The species has been studied extensively, both within its native range and in the regions of western Europe, South Korea and Japan it has invaded. Its colonies, founded by a single mated queen, become more populous as Summer progresses. By late Summer, its large, round papery nests, usually concealed by leaves in tree-tops, contain hundreds of worker hornets. To feed their larvae, they capture a wide variety of insects, with the Western Honey Bee (Apis mellifera) being a favored prey species, in part because stationary honey bee colonies provide food consistently for weeks on end (Fig. 1; Roy, 2023). Laurino et al. (2020) provided a review of the biology of the species and its invasion of Europe, where programs to control the species cost millions of dollars annually.
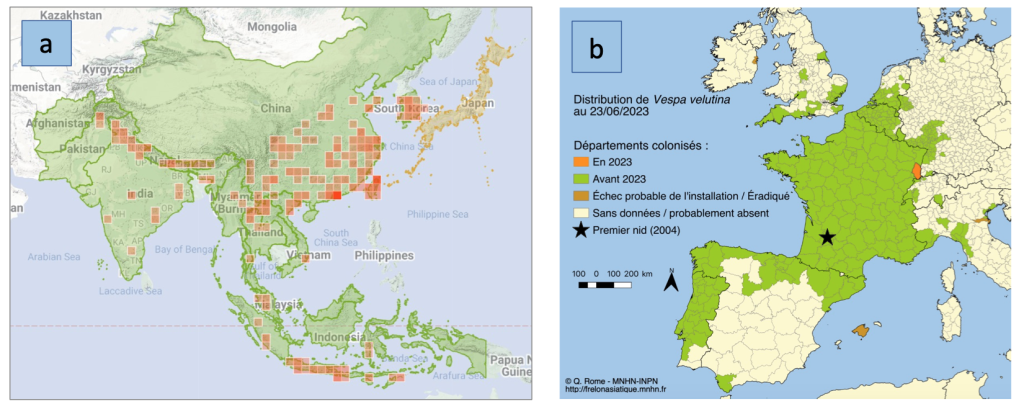
Figure 2. Distribution of V. veluntina (a) in Asia where it occurs naturally and has been introduced to South Korea and Japan, and (b) in Europe where it has been introduced. The red dot indicates where the first hornets were discovered in 2004. (Maps from (a) iNaturalist.org and (b) INPN (2023), used with permission of Quentin Rome / MNHN
Vespa velutina naturally inhabits a region that extends from northern China south through Indonesia and Southeast Asia, and westward along the foothills of the Himalayas to Afghanistan (Fig. 2a). Over that range, it occurs in 13 different color forms (formerly referred to as subspecies). It is likely that the hornets in Georgia are of the northern mainland form, V. velutina nigrothorax, that has also invaded Europe (Fig. 2b) and northeastern Asia. V. v. nigrothorax has often been inappropriately referred to in much of the literature written about it as the “Asian hornet”, a misnomer given that all 22 species of hornets inhabit some part of Asia!
The life cycle of the Yellow-legged Hornet is generally the same as that of all species of hornets (i.e., wasps in the genus Vespa) in temperate climates (Laurino et al., 2020). A mated queen emerges from her wintering site and becomes active in Spring when the weather becomes suitable. After a period of feeding on floral nectar and tree sap, followed by dispersal estimated to exceed 30 miles (50 km) in some instances, she individually constructs a small comb enclosed within a papery envelope made from plant fibers she chews and mixes with her saliva. The queen then rears the first generation of hornets by herself in an “embryo nest.” When those first workers have completed their development and emerge as adults, they take over most colony activities (nest construction, brood care, foraging and colony defense) while the queen continues to lay most, if not all, eggs. Towards Fall, the colony rears new queens and males. Those new queens mate with males, search for a wintering site that is usually in soil, leaf litter or rotten wood, and become quiescent for several months while temperatures are too cool for activity. It is while they are in Winter diapause that the queens can be accidentally transported long distances as stowaways among cargo on ships and, less frequently, on planes. This is almost certainly how Yellow-legged Hornets arrived in Georgia: the sites where the first individuals were discovered are within 12 miles of the Port of Savannah, one of the largest shipping ports in North America that receives cargo from everywhere in the world.
One important difference between the Yellow-legged Hornet and other species is the relatively large number of males with which young queens mate. The majority of hornet species that have been studied, mate with one or sometimes two males. In contrast, V. velutina queens mate with three males on average (range from one to 5+). The invasion of Europe by this species is believed to have originated from a single queen, mated with four males, that was accidentally imported to southwestern France (Fig. 2b). The added genetic variability provided by mating with several males has undoubtedly contributed to its successful invasions (reviewed by Otis et al., 2023).
Vespa velutina colonies can become very large. Quentin Rome and his colleagues (2015) collected nests in southwestern France from June to November and quantified their populations of immature and adult hornets. The colonies, generally initiated in March, had an average of 440 worker hornets by late October and early November. Some of those nests remained very small, but the most populous colony had 1,740 workers (News reports that colonies may contain 6,000 or more workers are erroneous). Mature colonies in Fall contained an average of 190 new queens (Rome et al., 2015), but because young queens only remain in their nest for about two weeks, that number must represent only a portion of all queens reared. I estimate that the average number reared is approximately 300–400 per colony. The greatest number of new queens collected inside one single nest was 463.
In an odd quirk of biology, hornet larvae serve as reservoirs of food within their colony. Larvae convert the proteins in chewed-up insect prey collected by worker hornets into amino-acid rich sugars. They then feed those secretions to young queens, resulting in them increasing in weight by 20–40% within one to two weeks of emergence as adults due to deposition of fat that fuels their survival during several months of Winter diapause. Young males also require the nutrients provided by larvae in order to mature sexually. Because hornets do not store honey in their nests, the sugary secretions from larvae also sustain their colonies during periods of inclement weather when foraging is not possible (reviewed by Otis et al. 2023).
How does the Yellow-legged Hornet affect honey bees and other pollinators?
The greatest concern related to the establishment of Yellow-legged Hornets in North America is their effects on honey bees and other pollinators. These hornets capture a wide variety of flying insects, a high percentage of which are pollinators, to feed to their larvae. Honey bees in particular are heavily preyed upon. Hornets hover near a hive entrance, facing outwards, so they can pick off incoming foragers (Fig. 1). Once a hive has been located, a hornet can return repeatedly to prey upon the essentially defenseless honey bees. Dr. Yves Le Conte, former head of the French honey bee research unit in Avignon, told me that by late Summer, when honey bees colonies should “prepare good Winter bees and collect honey for Winter survival, the hornets put pressure on the bees.” Hornets seem to focus on weaker colonies and in some cases, once they have killed most of the bees in a hive, they may enter and eat both the brood and the honey. In addition to the constant attrition of foraging bees, the presence of multiple hornets at the entrance of a colony causes “foraging paralysis” in extreme cases, flight activity is completely suppressed. The reduced foraging results in low honey stores and Winter starvation, with losses of 30–50% often reported (Yves Le Conte, pers. comm.; Requier et al., 2019). This hornet is a very significant threat to honey bees!
What has happened in Georgia in 2023?
From the information I have been able to obtain, Yellow-legged Hornets were first observed on or before August 1st by Sarah Beth Waller (personal communication), the beekeeper at the Savannah Bee Company on the eastern edge of Savannah where they were feeding on fallen pears. She posted a photo to iNaturalist on August 7th that yielded a tentative identification of the hornet as Vespa velutina (iNaturalist, 2023). Also in early August, a beekeeper somewhere in Savannah (at a date and location that have yet to be revealed by the Georgia Department of Agriculture) collected two hornets as they visited his hives. They were identified first by a University of Georgia entomologist and subsequently confirmed by the USDA on August 9th (GDA, 2023a). Thanks to news reports on August 15th about this discovery, a homeowner in the vicinity of the Savannah Bee Company reported a large nest 75 feet up in a pine tree. That nest, destroyed on the evening of August 23rd, proved to be exceptionally large (GDA 2023a, b). Keith Delaplane (University of Georgia) informed me that hornet traps of an unknown type have been deployed by the GDA from Port Wentworth to the mouth of the Savannah River, a distance of 18 miles. One person I interviewed was of the understanding that the first two locations where the hornets were observed are approximately eight miles apart, which, if true, would suggest that additional colonies may be present (hornets usually forage within a mile of their nest). Hornets have been sent for genetic analysis to determine if they originated in Europe or Asia. The situation in Savannah is fluid and changing rapidly. By the time this article is published, much more will be known and divulged. It may take a year or more before we know whether this incipient infestation has been eradicated.
What can be done to reduce the threat posed by the Yellow-legged Hornet?
Traps of many designs have been developed and tested in Europe. They have proven moderately effective for monitoring the presence of the hornet, but do not catch sufficient hornets to appreciably affect established colonies. Moreover, they have been criticized for their extensive by-catch of non-target insects. If the traps that have been deployed in Georgia capture additional hornets, finding and destroying their nests before new queens disperse into the environment presents the greatest probability of successful eradication. As an example, in spite of repeated discovery of Yellow-legged Hornet colonies in southern England, coordinated efforts of agricultural personnel, beekeepers and scientists have so far been successful at preventing the wasp’s establishment in the United Kingdom (Jones et al., 2020). On the Mediterranean island of Mallorca, the combination of Spring trapping of gynes before they establish nests, baiting for foragers, and triangulating and destroying colonies before they produce new queens, eliminated the initial infestation. There have been some successes in using very light-weight radio transmitters to track Vespa hornets to their nests (Kennedy et al., 2018; Looney et al. 2023). After a few years, however, once a population of hornets has become established, the proportion of nests that can be found and destroyed has proven in several European countries, to be insufficient to control the invasion.
Monitoring by state departments of agriculture (e.g., see the online form set up for reporting in Georgia; GDA 2023a) or through community-scientist platforms such as iNaturalist can provide accurate reporting of exotic hornets and other pests. Beekeepers can play a huge role in early detection of non-native hornets because several species that have the potential to become established in North America prey extensively on honey bees. Any wasp that seems unusual can be photographed or caught in a jar, then compared with similar species (USDA-APHIS, 2023). It should be reported if you suspect it is V. velutina or another non-native species.
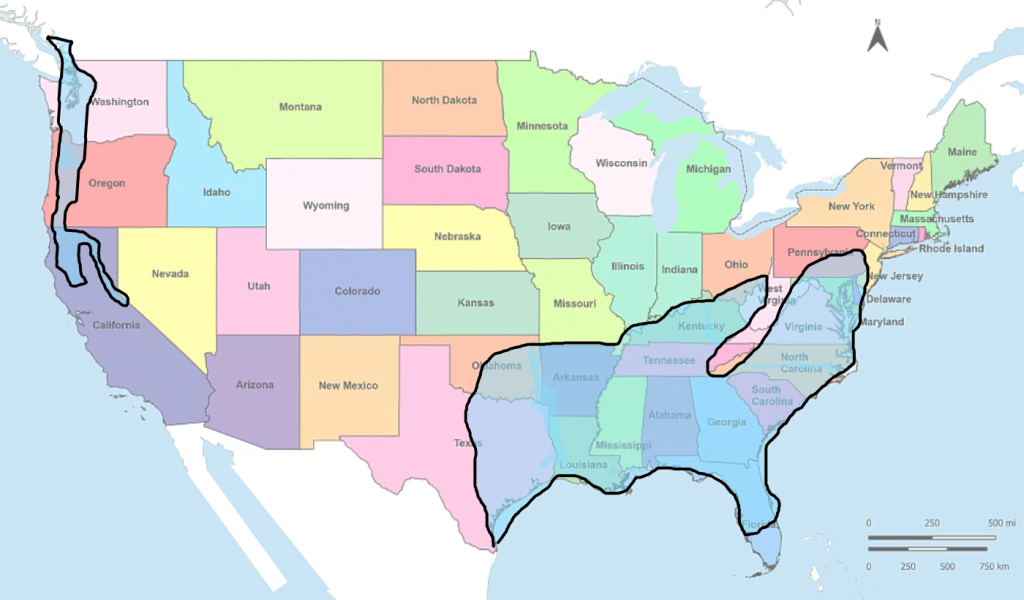
Figure 3. Regions of North America with climate well suited to Yellow-legged Hornet survival. Other regions with lower climate suitability not shown. Adapted from Villemany et al. (2011).
Where is the Yellow-legged Hornet likely to survive in North America?
GIS technology coupled with climate data for localities throughout the world that are available online have revolutionized our ability to predict potential distributions of species outside of their native ranges. In the case of the Yellow-legged Hornet, climate variables from where the species has been documented in Asia as well as in its introduced range in France, were analyzed along with climate data for sites throughout the rest of the world. A map showing the probability of suitable climate for hornet survival was created (Villemant et al., 2011). I superimposed the North American portion of that map onto a map of the United States, then modified it to show the approximate regions in North America where Vespa velutina would almost certainly be able to survive and reproduce (Fig. 3).
A large area of southeastern USA is predicted to have climate suitable for V. velutina. That region extends from the southern tip of Texas north into Oklahoma, then eastwards to somewhere between Baltimore and New York (Fig. 3.). Hornet survival may even be possible as far north as Boston and southern Ontario (Villemant et al., 2011). On the west coast, there is a zone in the rain shadow of the coastal mountains, from Vancouver, Canada, south into California, that seems prone to invasion. The hornet is likely to do better where the minimum temperature in Winter is not too cold, relative humidity is fairly high and the maximum temperature in Summer is not too hot.

Figure 4. The Northern Japanese Hornet, Vespa mandarinia, a species that invaded the Pacific Northwest in 2019. Photo taken by Aline Horikawa near Kyoto, Japan, and used with her permission.
What about other exotic species of hornets?
Who can forget the “invasion by murder hornets” in 2019–2021? The Northern Giant Hornet (formerly known as the Asian Giant Hornet), Vespa mandarinia, is a huge social hornet (Fig. 4; also, refer to the image within YLH lookalikes” in USDA-APHIS, 2023) that is infamous for its ability to rapidly slaughter entire colonies of Apis mellifera. Its discovery in Fall of 2019 led to an extensive monitoring and eradication effort that is on-going in Washington state and British Columbia. Following a peak of 35 sightings and specimens in 2020, there were only 10 reports in 2021 (Looney et al., 2023). Since then, there have been no Northern Giant Hornets detected in North America, and there is optimism that the introduction of this species has failed. It is not clear what combination of factors is responsible for the decline in its population: relatively unsuitable climate, low genetic diversity, destruction of nests (one in British Columbia, four in Washington state; Looney et al. 2023) or other factors. It is known that relatively few species that reach foreign lands are successful in establishing permanent populations. We may simply have gotten lucky with the recent Northern Giant Hornet introduction.
Several groups of researchers have modeled the potential distribution of V. mandarinia. All of them yielded a strong probability of it surviving in the Pacific Northwest where it was initially discovered as well as a large region of eastern North America; however, these models fail to agree on the regions in the east that are most prone to invasion. Because this species focuses its predation in late Summer and Fall on social insects, including honey bees, beekeepers are again the group most likely to encounter it. If you see any wasp attacking and killing honey bees, you should report it immediately.

Figure 5. The Oriental Hornet, Vespa orientalis, a demonstrated invader in northern Italy, southern France, Sardinia, southern Spain, eastern Europe and Chile. Photo taken by Nicola Addelfio near Palermo, Sicily, Italy, and used with her permission.
The Oriental Hornet (Vespa orientalis: Fig 5.), naturally inhabits the Mediterranean, Middle Eastern and western Asian countries and is the only hornet species adapted to hot, arid climates. This striking reddish brown hornet is an agricultural pest that attacks honey bee colonies and damages fleshy fruits such as grapes. It has already been detected in at least 10 countries outside of its native range (reviewed by Otis et al., 2023), and has successfully colonized southern Spain and Chile. Young queens often Winter in groups (Eran Levin, pers. comm.), a behavior which may have helped it to overcome genetic bottlenecks. Species distribution modeling suggests it has a high probability of successfully colonizing the Gulf Coast region of the United States as well as central California.
I would be remiss not to mention the European Hornet, also a large wasp that could be mistaken for one of the other species mentioned (USDA-APHIS, 2023). It was accidentally introduced to New York City nearly 180 years ago and has become relatively common in much of Eastern North America, but because it rarely captures honey bees and has relatively small colonies, it has not proven to be a concern for beekeeping.
Conclusion
Several hornet species cause extensive damage to honey bee colonies in other parts of the world. Unlike Varroa mites and small hive beetles, hornets do not inhabit bee colonies and would be very unlikely to be transported with hives when they are moved for pollination. However, they do have the potential to arrive at any port and subsequently be transported by trucks and trains anywhere in North America! The establishment of any of the exotic hornets discussed before would cause extensive disruption to beekeeping due to their predation on bees and secondary effects on Winter colony survival. The propensity of these hornets to attack honey bees makes beekeepers the most likely people to encounter them, as demonstrated recently with the detection of the Yellow-legged Hornet in Georgia. Readers are encouraged to learn how to identify them: review the figures in this article and “lookalikes” in USDA-APHIS (2023). Stay vigilant—and let’s hope that we remain “hornet-free” for many years to come.
Acknowledgments
I am indebted to Lien T.P. Nguyen of Vietnam and Heather Mattila of Wellesley College, MA, my hornet research collaborators, for the knowledge and experiences they have shared with me over the past decade. Staff at the University of Georgia, K. Cecelia Sequira of USDA-APHIS, and Sarah Beth Waller shared important information about the Yellow-legged Hornet detection in Georgia up until 27 August, 2023. I must also recognize the dozens of wasp biologists worldwide who have willingly answered my many naive questions about hornets.
Gard W. Otis
School of Environmental Sciences, University of Guelph, Ontario, Canada
Institute of Bee Health, University of Bern and Agroscope, Bern, Switzerland
Selected References
GDA (2023a). Georgia Department of Agriculture press release (Aug. 25, 2023). https://agr.georgia.gov/pr/yellow-legged-hornet-nest-eradicated-savannah-area
GDA (2023b) Georgia Department of Agriculture press conference (Aug. 25, 2023). https://www.facebook.com/watch/live/?ref=watch_permalink&v=639982398259487
iNaturalist (2023). Yellow-legged Hornet (Vespa velutina). https://www.inaturalist.org/taxa/119019-Vespa-velutina
INPN (2020). Le frailon asiatique, Vespa velutina. (The Asian Hornet, Vespa velutina). Inventarie National du Patrimoine Naturel. (http://frelonasiatique.mnhn.fr/)
Jones E.P., C. Conyers, V. Tomkies, et al. Managing incursions of Vespa velutina nigrithorax in the UK: an emerging threat to apiculture. Scientific Reports (2020) 10:19553. doi: 10.1038/s41598-020-76690-2
Kennedy P.J., S.M. Ford, J. Poidatz, et al. (2018). Searching for nests of the invasive Asian hornet (Vespa velutina) using radiotelemetry. Communications Biology 1: 88. doi: 10.1038/s42003-018-0092-9
Laurino D, S. Lioy, L. Carisio, et al. (2020). Vespa velutina: an alien driver of honey bee colony losses. Diversity (2020) 12: 1–15. doi:10.3390/d12010005
Looney, C, B. Carman, J. Cena, et al. (2023) Detection and description of four Vespa mandarinia (Hymenoptera: Vespidae) nests collected in Washington State, USA. Journal of Hymenoptera Research 96: 1–20. https://doi.org/10.3897/jhr.96.99307
Otis, G.W., B.A. Taylor, and H.R. Mattila (2023). Invasion potential of hornets (Hymenoptera: Vespidae: Vespa spp.). Frontiers in Insect Science 3: 1145158. https://doi.org/10.3389/finsc.2023.1145158
Pérez-de-Heredia, I, E. Darrouzet, A. Goldarazena, et al. (2017). Differentiating between gynes and workers in the invasive hornet Vespa velutina (Hymenoptera, Vespidae) in Europe. Journal of Hymenoptera Research 60: 119–133. https://doi.org/10.3897/jhr.60.13505
Requier, F., Q. Rome, G. Chiron, et al. (1019), Predation of the invasive Asian hornet affects foraging activity and survival probability of honey bees in Western Europe. Journal of Pest Science 92: 567–578.
Rome, Q., F.J. Muller, A. Touret-Alby, et al. (2015). Caste differentiation and seasonal changes in Vespa velutina (Hym.: Vespidae) colonies in its introduced range. Journal of Applied Entomology 139: 771–782. https://doi.org/10.1111/jen.12210
Roy, H. (2023). BBC. Asian hornet guide. https://www.discoverwildlife.com/animal-facts/insects-invertebrates/asian-hornets-guide/
USDA-APHIS (2023). Yellow-legged Hornet. https://www.aphis.usda.gov/aphis/ourfocus/planthealth/plant-pest-and-disease-programs/honey-bees/yellow-legged-hornet (accessed 25 August, 2023).
Villemant C., M. Barbet-Massin, A. Perrard, et al. (2011). Predicting the invasion risk by the alien bee-hawking yellow-legged hornet Vespa velutina nigrithorax across Europe and other continents with niche models. Biological Conservation 144: 2142–50. doi: 10.1016/j.biocon.2011.04.009
Click Here if you listened. We’re trying to gauge interest so only one question is required; however, there is a spot for feedback!
Read along below!
 The Quiet Evolution of Apiary Mowing
The Quiet Evolution of Apiary Mowing
A Necessary Aspect of Apiary Management
By: James E. Tew
My First Real Job
When I was a young teenager, a friend and I would push our mowers around the community and offer to cut grass. We generally earned somewhere between $1.75-2.50 per yard total. We had to split the earnings. The mowers were not self-propelled. Consequently, as young entrepreneurs, we never had a body weight problem.
We developed a regular customer list and at our peak, we were cutting about twenty lawns per week. General expectations were that we cut in straight lines and tips were not offered. When we were thirsty, we drank water from faucets plumbed from the house. Dad provided the mower and the gas, but I was allowed to keep my earnings. Of course, he got his lawn cut for free.
To this day, I cut grass in bullet-straight lines, and I only mow when the lawn absolutely needs it. Now, throughout every Summer month, I marvel at the equipment that professional mowing services have and I compare all that modern equipment to the absolute minimal equipment that my friend and I used all those years ago. Times change, don’t they?
Before Gasoline Mowers
Before gasoline mowers became widely available, lawns were cut using various manual methods and tools. The common methods used for lawn maintenance before gasoline mowers were scythes, sickles, weed slings, grazing animals, manual push mowers and scissors. None of these options were feasible without manual labor. Even grazing animals required fence installation. The invention of gasoline-powered mowers modernized lawn care and made it much more efficient and accessible for homeowners, landscapers and even beekeepers.

Figure 1. A Kansas beeyard in 1920. Note beekeeper in the lower left of the photo who is wearing a vintage Alexander veil and gauntlet gloves.
What Did this Mean for Apiaries?
It means that our apiaries, decades ago were weedier, and more unkempt by today’s standards. I cannot find information indicating that grazing animals were common methods for foliage management in pre-mower days. No doubt, cows, horses and sheep would occasionally knock over hives or scratch against them.
In the past, all apiary grass and weeds were cut manually requiring hot labor commitments. I’m old enough to remember life before string trimmers and herbicides. Everything was weedier then.

Figure 2. In 1922, in this Iowa beeyard, all grass was cut using manual methods. Gasoline mowers, of the day, were heavy and uncommon.
To Add to the Laborious Task
In years past, just as the present, protective bee gear was commonly worn when cutting and pruning near bee hives. Wearing protective equipment was just as clumsy now as it was years ago. The requirement to wear protective clothing has always made apiary weed control a hot, tiring job. Thankfully, modern protective equipment is better ventilated and more comfortable, but it is still hot work.
No-Mow May
Due to my early years of incessantly cutting grass, as a senior citizen, I am now a reluctant lawn mower. To the chagrin of my neighbors whose lawns are always neatly manicured, I only mow when I must. In this way, I avoid needless mowing sessions and my bees have access to the clover and dandelions in my lawn.
When I first heard of the concept, I readily embraced the notion of a “No-Mow May” in which we just give our lawns a month off from trimming. I quickly found out that a mow-less May lead me directly into a hellish June, with tall grass that frequently required raking after cutting. I had to go back to the lawn maintenance drawing board.
String Trimmers in the Apiary
I don’t remember the first time I used a string trimmer. With my mowing history that I touted before, that memory void seems strange to me. But I do know that a string trimmer became a necessary component of the equipment that always went with me to an outyard. String trimmers are now a common, if unexciting, beeyard management tool. What’s their story?
A Short History of String Trimmers
The history of string trimmers, also known as weed whackers, weed eaters or line trimmers, dates to the early 1970s. The concept of using a rotating nylon string to trim grass and weeds emerged as an alternative to traditional lawn mowers and manual cutting tools.
The concept of a rotating nylon line for cutting vegetation was developed in the late 1960s by George Ballas, a Houston-based entrepreneur. He got the idea while watching the revolving brushes at a car wash (I find this interesting. The common safety razor was envisioned after a visionary watched a woodworker use a common hand plane. The flail honey comb uncapper was conceptualized as another visionary watched the conveyor belt perform at a grocery store checkout. Shouldn’t we all be more observant?). In 1971, he received a patent for his invention, which consisted of a fishing reel with fishing line attached to the spool. Ballas’ invention was the foundation for the modern string trimmer.
In 1972, George Ballas partnered with Jim Goad, an engineer, to refine the design and create the first commercial string trimmer. They established the Weed Eater company and introduced the first gas-powered, handheld string trimmer to the market. This model quickly gained popularity due to its effectiveness in trimming grass and weeds in hard-to-reach areas.
In the late 1970s and early 1980s, electric string trimmers began to appear on the market. These models were lighter and quieter than their gas-powered counterparts, making them more appealing to homeowners with smaller yards.
Bees and Trimmers
No matter how useful power mowers and trimmers may be, on some mowing days, the bees seem to despise them. Most experienced beekeepers have seen this defensive behavior. In fact, common management recommendations warn the beekeeper to expect this attack. It is thought that the odors and vibrations from the mowers and trimmers agitate the bees.
In my own experience gained when trimming around hives, it seems that the bee response is greatest during Summer months when a nectar dearth has ended and the colonies are at full populations.
I have never used a battery-powered trimmer, but I am sure some of you have. I ask if you have noticed less of a response when using battery-powered mowers and trimmers? Does their quietness and lack of fumes have a more lenient effect on the colonies?
Beekeepers and Trimmers
At this moment, I have two, hand-held string trimmers. I have one modified with a cutting blade for heavy or tough growth. Brambles, such as multiflora rose, are a challenge for either type of cutting head.
Even though I frequently use them, I increasingly have issues with string trimmers. The evolving issue is that the older I become, my trimming sessions grow shorter and shorter. My shoulders ache. I get noise warnings from my Apple watch. I get hotter and hotter in the protective gear that I must wear. I simply can’t do the job the way I once could.

Figure 3. An apiary with uncontrolled grass growth.
Consequently, I have grown to dread the task more and more. All the while, the grass and weeds have continued to grow. Out of necessity, I developed a tolerant attitude of tall grasses and weeds in my apiary. I put my hives on firm hive stands that were twenty inches from the ground and I kept the entrance free of tall weeds. Even then, the grass and weeds in my beeyard continued to grow. My bees seemed unphased by the tall grass in the yards, but increasingly, it became apparent that this approach could not last. Why?
Two reasons that altered my laissez-faire system of yard maintenance evolved. The first reason was you, the reader of Bee Culture articles. Photos and videos that I captured in my apiary looked terrible. For instance, while I wanted to write about a new swarm that I just acquired, my photo of the new bee hive was marred by tall grass and the appearance of a generally unmanaged area. (If you have back issues of Bee Culture, you can readily see these photos.) I grew afraid that you, the reader, would not understand the bigger picture.

Figure 4. A manicured yard that uses herbicides, grazing animals and electric fencing to keeps foliage at bay. C. Parton Photo
Secondly, the tall weeds made it difficult for me walk while carrying a super of honey or other related bee equipment. Briars tugged at my suit. Tall grass made me stumble as I walked. Grass grew in and around my unused equipment. As with the No-Mow May scenario that I discussed earlier, I had to return to the drawing board. I was physically unable to trim my entire beeyard with a string trimmer and it was too much to ask of my 1972 Snapper push mower to systematically mow this tall grass.
A Heavy Duty, Walk-Behind Trimmer
I would occasionally see advertisements for various models of walk-behind trimmers. I asked around my circle of beekeeping friends, but no one had experience with these machines. I checked online. Yes, wheel kits were available for my string trimmers. In theory, I could modify my handheld trimmers to be mobile. Again, I asked around my circle of beekeeping friends, but no one had experience with these wheel kits either. All the while, the grass continued to grow. Would a trimmer on wheels allow me to work longer and more consistently?

Figure 5. A walk-behind Cord Trimmer.
In mid-July, with apiary grass higher than my knees, I broke. This situation in my apiary was unacceptable and was never going to get better. I went to an equipment dealer to buy a wheel kit. They did not have one, but they did have a single walk-behind trimmer on the showroom floor. It was $400. I bought it on the spot. It uses four .175” spiral cutting cords and cuts a twenty-two-inch swatch. It cuts at five heights – from 1.5” to 3.5”. I set it to the highest setting. The machine fairly easily chewed through the tall weeds leaving me with a somewhat rough-looking finished job, but the weeds were readily cut down.
The nose on the machine does a reasonably good job of getting beneath my hive stands – not perfect – but reasonably good. The cords are not cost free and they do wear out, but the machine aggressively took out tall weeds. It worked.
The major drawback is that I must still push the machine through tall grass. That requires old fashioned perspiration, but it’s still easier than using a handheld string unit. Please know that I am not selling these units. I’m only looking for a yard maintenance remedy.
For beekeepers younger than or more physically fit than I am, a typical string trimmer would get the job done. As I have written in previous articles, I’m at a stage of my beekeeping where I try to put wheels on everything. String trimmers were no exception. I should also say that while the bees didn’t go crazy, I still needed to wear light protective gear when using the machine.

Figure 6. Weed whacking beneath hives.
Hot and Clumsy
This past July 2023 was the hottest July every recorded. Yet the grass kept growing. To keep the grass under control, grass-cutting beekeepers are hot, and clumsy, and are surrounded by irate bees. Can it get any worse? Yes, it can, at the same time, we are also using power mowing equipment. Occasionally, accidents happen. This is a beekeeper’s recent story.

Figure 7. Accidents happen quickly. Attacking bees can be distracting. Many of us have a story.
I got home from work and my wife wanted to cut the grass but she’d never used that particular riding mower. I changed out of my work boots into my Crocs and pulled the mower out of the garage for her. I decided to make a couple passes by my bee hives so my wife wouldn’t get stung.
My bees have never bothered me before. On the first pass, I had hundreds of bees come after me. They were stinging me so much that I was fearful I might have an allergic reaction. I quickly decided to jump off the riding mower and make a run for the house. My foot got caught in the pulley and belt on the mower deck. My croc stayed lodged in the mower belt while I ran into the house. When I got inside and got all the bees off me, I realized how badly my foot was hurt.
I went to the Emergency Room where they said it had broken my toe and cut a tendon. It almost cut my toe off. I had 12 stitches and had to wear a Draco shoe that keeps the weight on my heel and off my toe until my broken toe can heal.
Mowing is Not Beekeeping
Every apiary mowing situation is different but presently, we have an abundance of diversified mowing devices. That selection of devices does not mean that mowing is not hot, demanding work. Don’t go crazy cutting grass and weeds, but when you do mow, I would suggest wearing heavy shoes and a ventilated bee suit with a veil that opens to allow for water sips. Have a lit smoker at the ready. Mowing is not beekeeping. Pace yourself.
Thank you.
I appreciate you reading and sending any comments that you may have. Your time is valuable. I know that.
 Dr. James E. Tew
Dr. James E. Tew
Emeritus Faculty, Entomology
The Ohio State University
[email protected]
Co-Host, Honey Bee
Obscura Podcast
www.honeybeeobscura.com
Click Here if you listened. We’re trying to gauge interest so only one question is required; however, there is a spot for feedback!
Read along below!

Beekeeping
A Doorway to Nature
By: Ross Conrad
It has been suggested that a spiritual crisis is at the center of the long emergency we collectively face. This crisis manifests itself as a disconnect from the natural world and is considered by many to be one of the primary forces driving the growing degradation of environmental health. When we see ourselves as separate from the natural world, we view nature through the lens of how valuable it is to us personally, either economically or for its beauty. This is the typical Western approach to the notion of pristine wilderness. When we place such values on the natural world and its “resources” viewing it simply as a means to gain financial wealth or other material benefits, it can reinforce our separation from it.
Research indicates that people who have a strong emotional and spiritual connection to nature are more likely to behave positively towards the environment, wildlife and habitats. This suggests that nurturing a greater connection to the natural world among the general population may be critical in addressing our spiritual crisis and helping to reverse the current environmental emergency. There are many ways this nurturing of our connection can manifest including hiking and camping, fishing and hunting, farming and gardening, or bird watching.
For the readers of Bee Culture, beekeeping likely provides one of our primary windows into the natural world. Through beekeeping, we enter the fascinating world of the honey bee; from the waggle dance and the intricacies of swarm behavior, to honey bee biology and the production, use, and unique characteristics of the products of the hive. Our fascination with bees stems from our personal connection to them and our deep understanding of them and their ways. It has been claimed that the honey bee and beekeeping is the most studied and written about topic in the world, second only to us humans. The truth of course, is that all living creatures are absolutely fascinating: we just tend to be clueless to most of the wonder, beauty and amazing intricacies and relationships involved in the lives of the plants, animals and insects that surround us and that we may come into contact with. We simply don’t interact with them enough to understand them and their ways, as well as we do the honey bee, and this can result in their being under-appreciated.
The world of beekeeping acts as a doorway through which we are able to then connect with the wider natural world of all the pests, diseases, plants and weather patterns that impact our bees; for better or worse.
The truth is that we are not separate from nature and the earth. Our bodies are literally made of the same minerals of the earth; we live our lives on the earth surrounded by the natural world; and when we die our body goes back to the earth and eventually gets recycled by the natural world. What we do to the natural world, we do to ourselves. We may not die when a rare pollinator dies out and becomes extinct, but surely a small part of something within us dies, something sacred and precious.
A host of studies have pointed to the fact that the stronger our personal connection to the natural world, the greater our concern for the environment (Whitburn et al., 2019; Mackay and Smidtt, 2019). There is also strong evidence of a positive relationship between a person’s connection to the natural world and one’s personal health, wellbeing and happiness (Capaldi et al., 2014; Barragan-Jason et al., 2023). When individuals are exposed to natural environments, such as mountain tops, coastlines, meadows and forests, the exposure results in stress reduction and assists in mental recovery following intense cognitive activities. It has even been found that a hospital window view onto a garden-like scene can be influential in reducing patients’ postoperative recovery periods and analgesic requirements.

Beekeeping provides a doorway through which individuals can develop a strong spiritual connection to the natural world, especially those living in urban or suburban environments.
Embedded in diverse cultures around the world is the idea that people consciously and unconsciously seek connections with the natural world. The theory that this is a result of evolutionary history where humans have lived in intimate contact with nature was initially put forward by Harvard biologist and two-time Pulitzer prize-winner, E.O. Wilson, in the Biophilia hypothesis (Wilson, 1984). We humans appear to be innately attracted to other living organisms. Evidence suggests that this is particularly evident when life becomes difficult and stressful. How many of us can deny the relaxing effect of a quiet moment by a lake, the soothing effect of sitting by a river, the rejuvenation of a hike through a forest, a stress reducing stroll by the seaside, the calming effect of simply cuddling with a pet dog or cat, or spending time with the honey bee colonies in our apiaries. Simply put, we need contact with nature and the importance of our ability to connect with the natural world has only grown due to our increasingly urban, digital-screen and social media lifestyles that often serve to disconnect us from nature which in turn, may contribute to health and wellbeing problems.
One meta-analysis suggests positive short- and long-term health outcomes with improved self-esteem and mood with exposure to green environments. Proximity to water generated some of the greatest changes and the mentally ill experience the greatest self-esteem improvements (Barton and Pretty, 2010). Other researchers examining the link between finding meaning in life and our relationship to the natural world suggest numerous benefits that arise from a personal connection to the natural world. Not only does nature help us find meaning in life, it can enhance our appreciation for life, and how engaging in nature-based activities (such as beekeeping) “provides an avenue for many people to build meaningful lives” (Passmore and Krouse, 2023).
The idea that contact with nature benefits our mental and physical health appears to be strongly supported by the statistics. According to one researcher, “Animals have always played a prominent part in human life. Today, more people go to zoos each year than to all professional sporting events. A total of 56% of U.S. households own pets. Animals comprise more than 90% of the characters used in language acquisition and counting in children’s preschool books. Numerous studies establish that household animals are considered family members; we talk to them as if they were human, we carry their photographs, we share our bedrooms with them” (Frumkin, 2001).
Beekeepers have their own version of this in what is referred to as the “telling of the bees.” A tradition where it is believed that when the beekeeper dies, someone has to go tell the bees and perhaps hang a piece of black cloth on the hive to place it in mourning or else the colony would die out or abandon the hive. There appears to be many versions of this. Others tell the bees about important events in their lives particularly regarding a death in the family. Considering how easy it is for a beekeeper to put off caring for their bees with our busy lives, this tradition practically served as a way to keep the hives in the thoughts of those that survive a deceased beekeeper, so that they will hopefully prioritize finding a new custodian to take over responsibility for their care in a timely manner.
As a deep personal connection to the natural world, beekeeping has the potential to provide numerous benefits to its participants. Beekeeping encourages one to get exercise along with fresh air and sunshine, and there is significant evidence that suggests that even the occasional bee sting can help fortify the body’s immune system allowing it to more effectively deal with various ailments (provided of course that the person is not hyper allergic to honey bee venom). Beyond all this, we now know that beekeeping can also help establish a spiritual connection to the earth and all the life forms with which we share this planet; a connection that may be critical in our ability to effectively deal with our current reliance on damaging green-house gas emitting technologies that are slowly turning our lives and society upside down.
Many people are suggesting that the weather extremes we have been experiencing around the country and the world is the problem, when really the problem at its base level is the malevolent actions of individual people. Nurturing a greater connection to the natural world in greater numbers of people, such as through activities like beekeeping, might just hold part of the salvation for this world. Something to consider as you go about the business of caring for your bees this Autumn and are tucking your colonies in for the long Winter ahead.
Just the same as a month before,—
The house and the trees,
The barn’s brown gable, the vine by the door,—
Nothing changed but the hives of bees.
Before them, under the garden wall,
Forward and back,
Went drearily singing the chore-girl small,
Draping each hive with a shred of black.
Trembling, I listened: the Summer sun
Had the chill of snow;
For I knew she was telling the bees of one
Gone on the journey we all must go!
An excerpt from the poem Telling the Bees by John Greenleaf Whittier.
Read the full poem here: https://www.poetryfoundation.org/poems/45491/telling-the-bees
Ross Conrad is the author of Natural Beekeeping, Revised and Expanded 2nd Edition, and coauthor of The Land of Milk and Honey: A history of beekeeping in Vermont.
References:
Barragan-Jason, G., Loreau, M., de Mazancourt, C., Singer, M.C., Parmesan, C. (2023) Psychological and physical connections with nature improve both human well-being and nature conservation: A systematic review of meta-analyses, Biological Conservation, Volume 277:109842
Barton J, Pretty J. (2010) What is the best dose of nature and green exercise for improving mental health? A multi-study analysis. Environmental Science & Technology, 44(10):3947-55. doi: 10.1021/es903183r. PMID: 20337470.
Capaldi, C.A., Dopko, R.L., Zelenski, J.M. (2014) The relationship between nature connectedness and happiness: a meta-analysis, Frontiers in Psychology, Volume 5
Howard Frumkin, (2001) Beyond Toxicity: Human health and the natural environment, American Journal of Preventive Medicine, 20(3):234-240, ISSN 0749-3797, https://doi.org/10.1016/S0749-3797(00)00317-2
Mackay, C.M.L. and Schmitt, M.T. (2019) Do people who feel connected to nature do more to protect it? A meta-analysis, Journal of Environmental Psychology, 65:101323
Passmore, Holli-Anne and Krouse, Ashley, N. (2023) The Beyond-Human Natural World: Providing Meaning and Making Meaning, International Journal of Environmental Research and Public Health, 20(12):6170
Whitburn, J., Linklater, W., Abrahamse, W. (2019) Meta-Analysis of human connection to nature and proenvironmental behavior, Conservation Biology, https://doi.org/10.1111/cobi.13381
Wilson, E. O. (1984) Biophilia: the Human Bond with Other Species.: Harvard University Press, Cambridge, MA.
Click Here if you listened. We’re trying to gauge interest so only one question is required; however, there is a spot for feedback!
Read along below!
Found in Translation
Mite Drop!
By: Jay Evans, USDA Beltsville Bee Lab
Varroa mites remain the primary source of honey bee colony losses for beekeepers managing from one to 10,000 colonies. Scientists like us and ardent beekeepers are always on the hunt for new ways to reduce varroa damage to bees and their colonies. One intriguing strategy is to make mites simply fall off their adult bee hosts. Short of changing the electric charge of host or parasite, this repellency can come from 1) making hosts less grippy, 2) somehow clogging the incredibly strong tarsi (feet with ‘toes’ and a spongy, oily, arolia) of mites or 3) affecting mite behavior by making them less likely to find safe spots and hang on to their bees for dear life. Dislodged mites are far more vulnerable to hygienic worker bees and might also simply keep falling down to a hostless, hungry and hopefully, short life. This is probably a central reason that female varroa mites spend very little time wandering the combs of beehives unless they are moments away from entering the brood cell of a developing bee. While on adult bees, mites have much incentive to stay right there, whatever their host is doing to drop them.
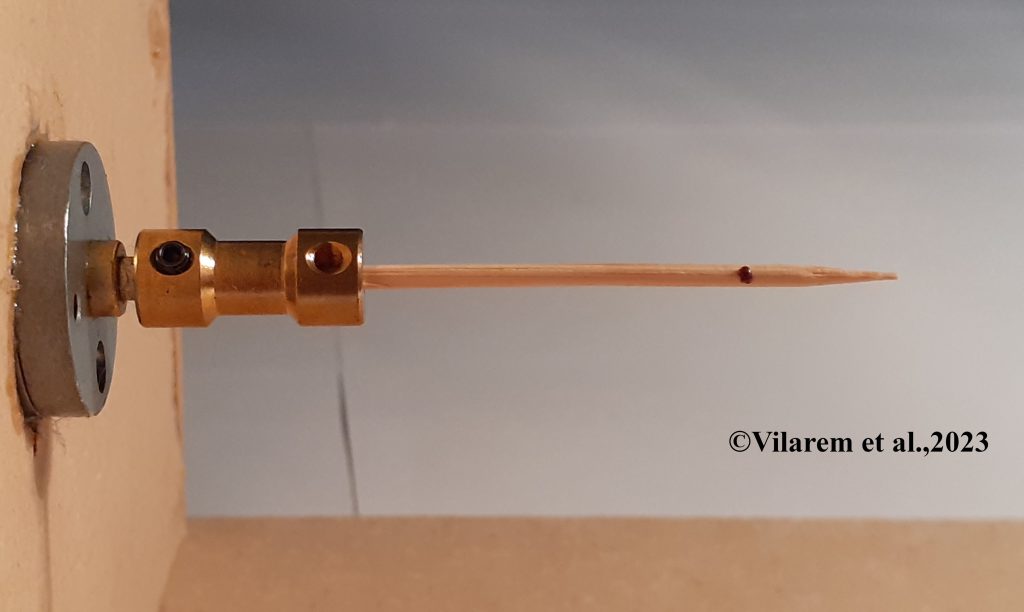 How do mites adhere to their bees so strongly? When mites are actively feeding on bees they are extremely hard to dislodge, since they are partly under the hardened plates of the bee itself and are gripping with a combination of ‘teeth’ and tarsi. Even while taking a break from feeding, mites know to find safe spots on the bee to attach, favoring locations on the abdomen or thorax that are both hairy and away from swinging legs and biting bee mandibles. How can one make them quit their bees given so many hiding places?
How do mites adhere to their bees so strongly? When mites are actively feeding on bees they are extremely hard to dislodge, since they are partly under the hardened plates of the bee itself and are gripping with a combination of ‘teeth’ and tarsi. Even while taking a break from feeding, mites know to find safe spots on the bee to attach, favoring locations on the abdomen or thorax that are both hairy and away from swinging legs and biting bee mandibles. How can one make them quit their bees given so many hiding places?
Caroline Vilarem and colleagues in France recently described an ambitious attempt to document the abilities of mites to hang onto surfaces when exposed to organic acids (Vilarem, C.; Piou, V.; Blanchard, S.; Vogelweith, F.; Vétillard, A. Lose Your Grip: Challenging Varroa destructor Host Attachment with Tartaric, Lactic, Formic, and Citric Acids, Appl. Sci. 2023, 13, 9085. https://doi.org/10.3390/app13169085). These scientists deployed one of the coolest low-tech tools to measure how well mites grip onto a surface. While their ‘Rotavar’ sounds both complex and expensive, it is actually a ‘motor-driven rotating toothpick’. Yes, you can do this at home, with a slow (three or so revolutions per minute) motor and a supply of toothpicks. The authors add to that an extremely careful experimental design and complex statistics to show the different abilities of mites to hang onto sticks and bees coated with acetic, citric, lactic, formic and tartaric acids. The results hint at new modes and new candidates for mite control, with the usual caveat that converting a controlled lab assay to field colonies will be challenging.
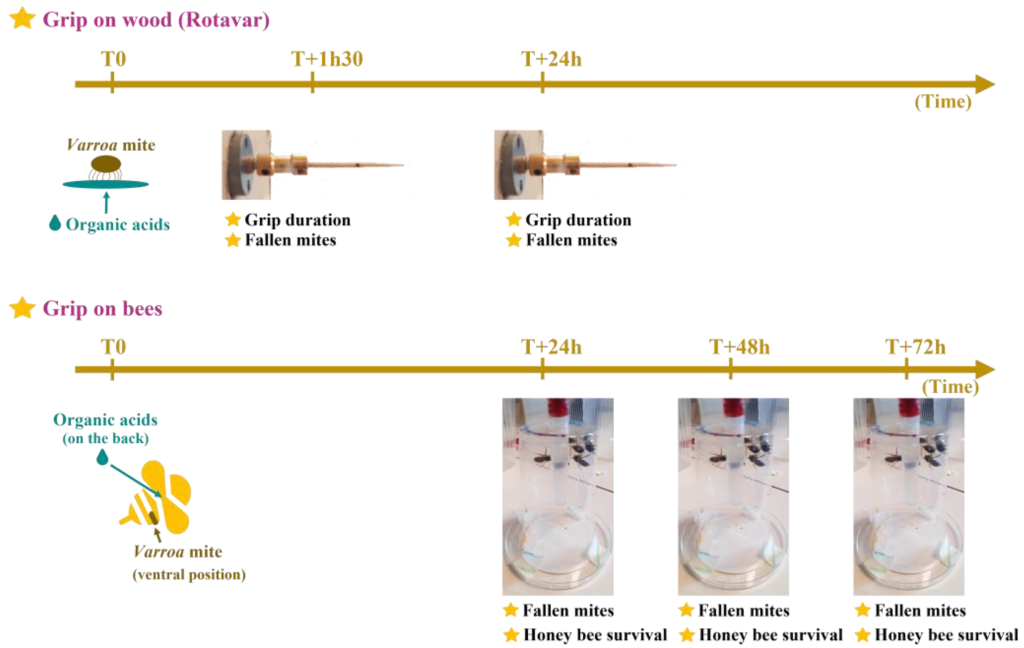
Schematic diagram of the experimental design and measured parameters. Grip on wood (Rotavar): This method relies on direct contact between Varroa’s arolia and the organic acids. The Rotavar set-up is a motor-driven rotating toothpick used to assess V. destructor’s grip. Grip on bees: the host attachment experiment applies acids to the backs of honey bees to remove mites. T0 represents the administration time for treatments; T + 1 h 30, 24 h, 48 h, or 72 h stand for the time post administration used to make measurements. Figure from https://doi.org/10.3390/app13169085
Some highlights: First, acidity itself does not seem to be the solution. Most notably, even high doses of acetic acid had little impact on the abilities of mites to grab toothpicks and this candidate was quickly discarded. So, what can we glean from the differences between the tested acids? Tartaric acid worked great at dislodging mites from spinning toothpicks but was surprisingly poor at dislodging mites from bees. Prior work suggests that the mode of action for tartaric acid is, at least in part, toxicity towards mites. It is possible that the levels of tartaric acid needed to coat bees with a toxic dose are higher than they are on a relatively smooth and barren toothpick. Toothpicks also attract watery compounds (hydrophilic) while bees are coated with oils and are hence more water-repellent (hydrophobic). Maybe the availability of tartaric acid on toothpicks is higher than it would be on oilier bee bodies. Formic acid also worked much better on the wood surface than on bees, an intriguing insight for a well-used and effective mite control. Formic acid is also known to be directly toxic to mites and their cells, and the authors make clear that both direct toxicity and grippiness are clear and perhaps synergistic targets for mite control. The widely used miticide oxalic acid also wins by being directly toxic to mites at levels that are relatively safe for bees, demonstrating that there are many possible ways to turn organic acids into effective treatments.
Lactic acid came out as the best candidate in the study group for divorcing mites from their bees. This acid worked well at dislodging mites from both toothpicks and bees. Lactic acid does not appear to be highly toxic to mites and instead seems to act by changing the mechanics of hanging on. This is a nice lead for exploring acids with similar qualities for their abilities to both grease the ‘Rotavar’ and make bees a more slippery host. In another intriguing result from this nice study, mites that simply walked across paper holding lactic acid were then less good in future grip tests. What is it about lactic acid that burns, cleans or otherwise insults the complex and surprisingly ‘soft’ tarsi of mites?
If this topic has gripped you, consider reading up on the field thanks to a recent open-access paper on stickiness by graduate student Luc van den Boogaart and colleagues in the Netherlands (van den Boogaart, L.M.; Langowski, J.K.A.; Amador, G.J. Studying Stickiness: Methods, Trade-Offs, and Perspectives in Measuring Reversible Biological Adhesion and Friction. Biomimetics 2022, 7, 134; https://www.mdpi.com/2313-7673/7/3/134). For those of us who have stored ‘Freshman Physics’ in a remote hard drive, they give a clear review of how these forces work across organisms; in their words ‘from ticks to tree frogs’. Maybe their figures and insights will inspire a beekeeper or scientist to dream up a safe, effective route to dislodge mites from bees and prevent them from climbing back on. Pulling in people with a knowledge of physics, or just really good imaginations and the ability to build and deploy Rotavars (imagine how entertaining those can be, a la squirrel spinners… https://www.youtube.com/shorts/nBKb_z4_tGY), can only help in the hunt for new mite controls and healthier bees.
]]>By: Nina Bagley
 Miss Lillian Love was born into a Quaker family in Marion, Indiana on October 24, 1880. Her family was from Decatur, Indiana. Her father, Granville Love, was born in Indiana. In 1860, he married Nancy J. Gillibrand. Her family came from England and settled in the vicinity of Indianapolis. The two were married on August 11, 1868, in Morgan County, Indiana. Granville was a farmer and ran a huckster wagon, which proved a good business. Mrs. Nancy Love had nine children from 1869 to 1892. She died on January 31, 1936, at eighty-six, in Decatur, Indiana. Lillian’s father, Granville Love, died May 7, 1925, in Guilford, Indiana. All the children would be trained in English and piano at Central Normal College in Danville, Indiana.
Miss Lillian Love was born into a Quaker family in Marion, Indiana on October 24, 1880. Her family was from Decatur, Indiana. Her father, Granville Love, was born in Indiana. In 1860, he married Nancy J. Gillibrand. Her family came from England and settled in the vicinity of Indianapolis. The two were married on August 11, 1868, in Morgan County, Indiana. Granville was a farmer and ran a huckster wagon, which proved a good business. Mrs. Nancy Love had nine children from 1869 to 1892. She died on January 31, 1936, at eighty-six, in Decatur, Indiana. Lillian’s father, Granville Love, died May 7, 1925, in Guilford, Indiana. All the children would be trained in English and piano at Central Normal College in Danville, Indiana.


Lillian’s parents, Granville and Nancy Love.
Lillian had two years of college, became a teacher, and taught in Indiana and Florida. Women still couldn’t find jobs other than teaching. Lillian and her youngest sister Flossie were involved in women’s rights and equal opportunity for women; they supported women’s rights to vote.
In 1904, Lillian moved to Tacoma, Washington, where she taught for several years. Finding her husband in 1907, she married Jay Levant Hill, who was twenty-five years older than her. Lillian’s husband, Jay, was an inventor who owned his own lumber business. Lillian said that her husband’s “brain was mechanically bent.”

Miss Lillian Love, taken in Washington State prior to her marriage to J. Levant Hill.
Their home would be Mount Shadow Ranch, a two-hundred-acre farm with a charming yellow California-style bungalow, two and a half miles from Elbe, Washington. Surrounding the farm were the bee’s favorite purple hills of fireweed.
Fireweed is a plant that enjoys cool and moist climates and thrives in Pacific Northwest forestlands. It is also considered one of the most prolific plants for honey production, with its nectar having a high sugar concentration. It has a “lightly spicy” or “buttery” flavor.
If you shut your eyes and listen, you can hear the train whistle in the distance as it stops at Park Junction Station in the middle of Mount Shadow Ranch.


1926 – Lillian Hill at Mt. Rainier
Apiary. Image featured in American Bee Journal
“We have some thirty acres under cultivation,” said Mrs. Hill; The rest of the farm is logged-off land which we use to pasture our herd of fifty cattle.”
—American Bee Journal, April 1926.
One day in 1913, an old man came peddling bees. “You have a wonderful place here for bees,” he said, convincing Mrs. Hill to invest in six swarms. Before the older man came along, she had never seen a swarm of bees before. Her six swarms increased and produced so much honey that in 1914 Lillian Hill invested in twenty more swarms, giving her forty hives. That year she harvested 6,500 pounds of pure honey. Lillian had an entrepreneurial spirit and determination to succeed in a male-dominated industry.

Lillian’s marriage to J. Hill.
I don’t know how she accomplished so much. Mrs. Lillian Hill kept a tidy home, raised beef cattle, Duroc-Jersey hogs, geese and ducks, and grew vegetables in the garden. But it would be the bees she loved the most!
Lillian was part owner of the Ranch and owner of the Mount Shadow Apiary. She had a gentle personality. She was independent and had a fire in her eyes that you could see demanded respect.
Being a novice beekeeper found her unprepared. In 1915, she encountered her first obstacle, European Foulbrood that would bring havoc to her beeyard. Words that no beekeeper wanted to hear or experience, the only cure, the dreadful burning of the hives. After that horrible experience with “American Foulbrood,” she only kept a dozen colonies of bees providing honey for her family and neighbors. (American Bee Journal, April 1926.)
“I never camp,” confessed Lillian Hill. “On either the trail of my successes or my failures. I go right on.” That’s her philosophy in a nut-shell.
Although childless, she cared for the children from reform schools, orphan asylums or neighboring farms; she taught the boys and girls everything about beekeeping so they could pay their way through school. She believed that the best and safest way to help any human being is to help him help himself. Particularly, those who needed guidance and education.
In the 1900s, the U.S. was a diverse nation, and its children lived in various circumstances. For years, she had been the leader of the Boys’ and Girls’ Bee Club of Elbe. One of her boys won nearly $80.00 with his exhibits of bees and honey at the Western Washington Fair.
Lillian increased her hives to twenty-six to help one of her boys and it didn’t stop there!
In 1924, to help one of her girls through school, she invested in thirty more hives and loaned them to the girl. The girl lived next door to an abandoned schoolhouse on an acre of ground, which got Mrs. Hill thinking, “I could rent the schoolhouse and land from the school board.”

1921 – Freddie May with his siblings before they were placed in the Washington Children’s Home.
One day in 1924, a young man showed up at the Ranch. His name was Freddie May, and he was born in 1912 in Denver, Colorado. When he was eight years old, he lived in Wenatchee, Washington. His father abandoned the family, and their mother could not care for six children. The children were placed in the Washington Children’s Home in Wenatchee, Washington, in 1921.

Mount Rainier Apiary. Freddie May and Mrs. Lillian Hill.
Freddie somehow got his hands on a newspaper. He came across the ad for a permanent position in beekeeping work. Freddie wanted to learn about the beekeeping business under the leadership of Mrs. Lillian Hill, so he rode on “a bicycle” from Wenatchee, Washington to Elbe, Washington, a hundred and ninety five miles! He was energetic and full of fire and wanted to learn beekeeping.
Lillian took a liking to Freddie and wanted to help him make money to pay his way through school, so she furnished Freddie with plenty of bees on a commission basis of fifty-fifty. In four months, the Colorado cyclist made five hundred dollars for himself.
Freddie would consider Mrs. Lillian Hill his mother and next of kin. Lillian and her husband would become Freddie’s foster parents giving him a home with security. He would attend Eatonville High School and work on the Ranch. He would continue beekeeping and eventually marry and have a family.
During the season of 1925, Mrs. Hill was able to establish the Colorado youth in the schoolhouse helping young boys and girls in need teaching them beekeeping.
In 1926, Lillian Hill had over one hundred and fifty hives of Italian bees, eighty-five at the schoolhouse and sixty-five at home. She produced at least 10,000 sections of comb honey. Mrs. Hill would advertise in the newspapers to get workers “Wanted – an experienced farmer for a permanent position.”
Most of the marketing she did herself in her Buick car. She supplied the best stores in Tacoma and Seattle. “I don’t have to hunt for a market,” declared this energetic woman. In one year, Mrs. Hill raised sixty queens. That was the part of her business that she enjoyed most of all. Mrs. Hill was the president of the Pierce County Beekeepers’ Association for two years.

Mount Rainier
Both triumphs and disasters have knocked often at Lillian Hill’s door on the Mount Shadow Ranch, but neither one ever fazed her. This woman had grit and plenty of it!
Around 1927, Freddie would accidentally run over Lillian’s foot crushing it while she was teaching him how to drive the tractor. An unfortunate outcome was that the doctors had to amputate her leg due to blood poisoning. Lillian had a prosthetic leg from the knee down, but that didn’t stop her. She took it in stride and persevered. In 1929, unfortunately, her husband died. He was the youngest of five and the last of his siblings. He was seventy-one years old. I will say some lives have more trial or tribulations than others, to be sure, but no life is without events that test and challenge us.
In the 1930 census, Lillian is listed as a widow forty-nine years old, with fifty men aged eighteen to sixty-six listed as boarders at the Mount Shadow Ranch and working for Lillian Hill. That’s a lot of men to manage. You would have to have grit and be firm! Among the fifty men working on the farm was Freddie May, the youngest, who was eighteen. His occupation was a Logger.
Not being able to care for the Ranch and losing her husband, not to mention the tractor accident, left her feeling like it would be time to sell the Ranch. Lillian Hill would place the Ranch up for sale.
Advertised in the Tacoma Daily Ledger Sunday, June 23, 1929. “Mountain Shadow Ranch. It is one of the best-stocked Dairy Farms in western Washington, with running water in every field and excellent soil. Forty acres cleared; 120 acres fenced for hogs and cattle; stocked and making money; good seven-room house with school buses to Elbe and Eatonville high school. The farm is a must-see to appreciate it. We will consider small trade—a price of $15,000. Write to Lillian L. Hill for an appointment.”

Family photo of Albert Cook, first wife Nora and their children.

Lillian’s family Bible.
A lot happened in 1930. The Ranch sold, and Lillian Hill married Albert Cook, a widower who worked in the lumber industry. His wife Nora passed away in March of 1929 at the age of fifty-one; they had six children together. Lillian didn’t mind an extended family. She was raising her niece Esther who she adopted at a young age and her foster son Freddie May. After all, Lillian loved children and teaching. Her first husband was in the lumber business so she probably knew Albert Cook.
Albert would marry Lillian in 1930, build apartment buildings and retire from the lumber industry. The two would live in Tacoma, Washington. Lillian’s beekeeping days came to an end, her new occupation would be owner and landlord of her apartment buildings.
Lillian had a very loving relationship for nineteen years with her husband Albert. In May of 1949, Albert passed away at the age of seventy-three. He was buried beside his first wife, Nora, in Tacoma, Pierce, Washington.
The income from the apartments and other investments would give Lillian a comfortable life for the next sixteen years. She lived to be eighty-eight and passed away August 19, 1969 in Tacoma, Pierce, Washington Lillian was a Sixth Avenue Baptist church member. She was buried next to her first husband, Jay Levant Hill.
Freddie May lived to be eighty-three years old. Freddie kept his surname May. He went by Fred (Cook) May, Sr. “Commander” as best by everyone who loved him.

Lillian in her sister Flossie’s backyard. Her dress is purple and black print. She and her sister Flossie always had a matching rhinestone necklace.
Lillian’s youngest sister Flossie, who she remained close with, lived in California. Flossie had a granddaughter Karla who enjoyed her aunt Lillian’s visits. She remembers sitting on her grandmother’s hunter green “davenport” with her aunt Lillian. Her grandma Flossie would sit in her desk chair across the room and the two sisters would talk for hours.
Lillian’s great-niece Karla also remembers how “intriguing” her aunt was. Lillian had blue eyes, was fair-haired and had rosy cheeks. She wore her hair in a braid reaching her waist until one day; she cut it off, curled it up, and put it in a small box for keeping. Karla remembers her Aunt Lillian as sweet but at the same time, tough and gutsy!
Marcus Aurelius was a stoic philosopher. His quote reminded me of Mrs. Lillian Love, her struggles as a woman in the 1900’s and how she put others before her, passing her knowledge about beekeeping on to so many young boys and girls in need. I would like to thank Lillian’s great-niece Karla Babcock for sharing her memories of her Aunt Lillian and grandmother Flossie.
“A life of sacrifice and putting the well being collective first, just like the bees.”
—Marcus Aurelius
Ohioqueenbee
Nina M. Bagley
Columbus, Ohio.
 Fresh Broccoli with Honey
Fresh Broccoli with HoneyBy: Fay Jarrett
Ingredients
□ 4 cups chopped broccoli
□ ¼ cup honey
□ 1 tbsp olive oil (tuscan herb was used this time)
□ ½ tsp pepper
□ ½ tsp salt
Directions
Step 1
Place chopped broccoli in a large bowl.
Step 2
Sprinkle honey, olive oil, salt and pepper over top. Stir.
Step 3
Cook broccoli in the microwave for four to five minutes, until desired tenderness.
Note
This a great addition to pork chops with hot honey drizzled on top.
Enjoy this easy, delicious dinner!





 Nestmate Recognition
Nestmate RecognitionBy: Clarence Collison
Pheromones are involved in intraspecific chemical communication; however, the glands associated with compounds used in nestmate recognition in honey bees remain elusive. This search is difficult since nestmate cues can arise from both within the colony, and from the environment (Kalmus and Ribbands, 1952). For example, Downs and Ratnieks (1999) found no evidence that honey bee guards used heritable cues; instead, guards appear to rely exclusively on environmental cues to distinguish nestmates from non-nestmates. However, nestmate cues can also be produced by the individual, and thus must be under genetic control (Breed, 1983; Page Jr. et al., 1991). A further factor is that the wax used to build comb in the colony is both produced and manipulated by the bees, which means it may be a medium into which recognition cues are transferred (Breed et al., 1998). Therefore, Breed et al. (1998) stated that no single factor is responsible for nestmate recognition in honey bees; rather, all three factors (genetically determined cuticular signatures, exposure to comb wax, and environmental cues e.g. floral cues) seem to work together (Martin et al., 2018).
Comb wax in honey bee colonies serves as a source and medium for transmission of recognition cues. Worker honey bees learn the identity of their primary nesting material, the wax comb, within an hour of emergence. In an olfactometer, bees discriminate between combs on the basis of odor; they prefer the odors of previously learned combs. Representatives of three of the most common compound classes in bee’s wax were surveyed for effects on nestmate discrimination behavior. Hexadecane, octadecane, tetracosanoic acid and methyl docosanoate make worker honey bees less acceptable to their untreated sisters. Other similar compounds did not have this effect. These findings support the hypothesis that nestmate recognition in honey bees is mediated by many different compounds, including some related to those found in comb wax (Breed and Stiller, 1992).
Breed et al. (1998) investigated how kin recognition cues develop and cue differentiation between honey bee colonies. Exposure to the wax comb in colonies is a critical component of the development of kin recognition cues. In this study, they determined how the cues develop under natural conditions (in swarms), whether the genetic source and age of the wax affect cue ontogeny, and whether exposure to wax, as in normal development, affects preferential feeding among bees within social groups. Cue development in swarms coincided with wax production, rather than with the presence of brood or the emergence of new workers; this finding supported previous observations concerning the importance of wax in cue ontogeny. Effective cue development required a match between the genetic source of the workers attempting to enter the hive, the wax to which they were exposed and the guards at the hive entrance. The wax must also have been exposed to the hive environment for some time. Cues gained from wax did not mask or override cues used in preferential feeding interactions; this finding supports the contention that two recognition systems, one for nestmate recognition and the other for intra-colonial recognition, are present.
Recognition of nestmates from aliens is based on olfactory cues, and many studies have demonstrated that such cues are contained within the lipid layer covering the insect cuticle. These lipids are usually a complex mixture of tens of compounds in which aliphatic hydrocarbons are generally the major components. Dani et al. (2005) tested whether artificial changes in the cuticular profile through supplementation of naturally occurring alkanes and alkenes in honey bees affect the behavior of nestmate guards. Compounds were applied to live foragers in microgram quantities and the bees returned to their hive entrance where the behavior of the guard bees was observed. In this fashion, they compared the effect of single alkenes with that of single alkanes; the effect of mixtures of alkenes versus that of mixtures of alkanes and the whole alkane fraction separated from the cuticular lipids versus the alkene fraction. With only one exception (the comparison between n-C19 and (Z)9-C19), in all the experiments bees treated with alkenes were attacked more intensively than bees treated with alkanes. This led them to conclude that modification of the natural chemical profile with the two different classes of compounds has a different effect on acceptance and suggests that this may correspond to a differential importance in the recognition signature.
 Cuticular hydrocarbons (CHCs) function as recognition compounds in honey bees. It is not clearly understood where CHCs are stored in the honey bee. Martin et al. (2018) investigated the hydrocarbons and esters found in five major worker honey bee exocrine glands, at three different developmental stages (newly emerged, nurse and forager) using a high temperature GC analysis. They found the hypopharyngeal gland contained no hydrocarbons nor esters, and the thoracic salivary and mandibular glands only contained trace amounts of n-alkanes. However, the cephalic salivary gland (CSG) contained the greatest number and highest quantity of hydrocarbons relative to the five other glands with many of the hydrocarbons also found in the Dufour’s gland, but at much lower levels. They also discovered a series of oleic acid wax esters that lay beyond the detection of standard GC columns. As a bee’s activities changed, as it aged, the types of compounds detected in the CSG also changed. For example, newly emerged bees have predominately C19-C23n-alkanes, alkenes and methyl-branched compounds, whereas the nurses’ CSG had predominately C31:1 and C33:1 alkene isomers, which are replaced by a series of oleic acid wax esters in foragers. These changes in the CSG were mirrored by corresponding changes in the adults’ CHCs profile. The CSG is a major storage gland of CHCs. As the CSG duct opens into the buccal cavity (mouth), the hydrocarbons can be worked into the comb wax and could help explain the role of comb wax in nestmate recognition experiments.
Cuticular hydrocarbons (CHCs) function as recognition compounds in honey bees. It is not clearly understood where CHCs are stored in the honey bee. Martin et al. (2018) investigated the hydrocarbons and esters found in five major worker honey bee exocrine glands, at three different developmental stages (newly emerged, nurse and forager) using a high temperature GC analysis. They found the hypopharyngeal gland contained no hydrocarbons nor esters, and the thoracic salivary and mandibular glands only contained trace amounts of n-alkanes. However, the cephalic salivary gland (CSG) contained the greatest number and highest quantity of hydrocarbons relative to the five other glands with many of the hydrocarbons also found in the Dufour’s gland, but at much lower levels. They also discovered a series of oleic acid wax esters that lay beyond the detection of standard GC columns. As a bee’s activities changed, as it aged, the types of compounds detected in the CSG also changed. For example, newly emerged bees have predominately C19-C23n-alkanes, alkenes and methyl-branched compounds, whereas the nurses’ CSG had predominately C31:1 and C33:1 alkene isomers, which are replaced by a series of oleic acid wax esters in foragers. These changes in the CSG were mirrored by corresponding changes in the adults’ CHCs profile. The CSG is a major storage gland of CHCs. As the CSG duct opens into the buccal cavity (mouth), the hydrocarbons can be worked into the comb wax and could help explain the role of comb wax in nestmate recognition experiments.
Worker honey bees are able to discriminate between combs on the basis of genetic similarity to a learned comb. The nestmate recognition cues that they acquire from the comb also have a genetically correlated component. Cues are acquired from comb in very short exposure periods (five minutes or less) and can be transferred among bees that are in physical contact. Gas chromatographic analysis demonstrates that bees with exposure to comb have different chemical surface profiles than bees without such exposure. These results support the hypothesis that comb-derived recognition cues are highly important in honey bee nestmate recognition. These cues are at least in part derived from the wax itself, rather than from floral scents that have been absorbed by the wax (Breed et al., 1995).
Experiments indicated that the most important recognition pheromones are the fatty acids, particularly palmitic acid, palmitoleic acid, oleic acid, linoleic acid, linolenic acid and tetracosanoic acid. These fatty acids are mixed with the wax hydrocarbons from wax glands, molded into comb and then transferred onto the workers as they contact the comb. The result is a colony level signature that varies little among workers in a colony. Newly emerged workers have few external fatty acids or hydrocarbons. Oleic acid is more abundant than the other fatty acids on newly emerged bees, but the amount of oleic acid on the cuticle does not vary significantly among colonies. Newly emerged workers are accepted even though they have no signature yet; the “password” for new bees to be admitted to their colony is apparently the lack of a signal. This conclusion is corroborated by the finding that guards tend to treat sodium hydroxide-washed older bees as if they are newly emerged (Breed, 1998).
The integration of recognition cues is described as follows. Fatty acids and hydrocarbons are components of the wax comb that is produced by the bees. The relative abundances of fatty acids and hydrocarbons in wax varies among colonies, giving them unique chemical signatures. Food odors may also be absorbed by the comb, adding to its uniqueness. Newly emerged bees produce their own hydrocarbon coating, which is modified as they move around the nest by the addition of hydrocarbons and fatty acids from the comb. Of the compounds tested in the laboratory, fatty acids are the most important recognition pheromones, but other, as yet untested compounds may also contribute to the recognition odor. Hydrocarbons have generally been assumed to be the primary recognition pheromones of honey bees. However, none of the major structural hydrocarbons of honey bees (i.e., n-alkanes) yields a positive result in a recognition bioassay, nor do these compounds differ significantly in relative concentration among families of bees (Breed, 1998).
The environmental and genetic components of recognition are difficult to separate even in controlled conditions. Getz and Smith (1983) showed that the honey bee discriminates between full and half-sisters raised in the same hive, on the same brood comb in neighboring cells, thus demonstrating a significant genetic component to the recognition process.
Nestmate recognition information can come from either contact chemoreception or olfaction. Mann and Breed (1997) investigated what role airborne olfactory cues play in nestmate recognition by honey bee colony guards, and how do these signals affect guard orientation and behavior? They demonstrated that airborne cues play a significant role in guard bee recognition of nestmates and non-nestmates. Exposure of a guard bee to the scent of a non-nestmate resulted in increased locomotory rate and changes in the directional orientation of guard bees. Exposure to scent of a non-nestmate did not, however, increase the likelihood that a second non-nestmate would be attacked when placed with the guard. Observations of guard behavior at colony entrances indicate that guards discriminate nestmates from non-nestmates with high efficiency.
Floral oils are an important component of the honey bee’s olfactory environment. Bowden et al. (1998) used laboratory and field tests to determine whether floral oils affect nestmate recognition in honey bees. In the laboratory, newly emerged worker bees, that have not been exposed to comb wax, responded more aggressively to bees that had been exposed to floral oils than unexposed control bees. In the field, guard bees did not respond differently to foragers that had been exposed to floral oils. Floral oils may play a supplementary role in nestmate recognition; however, if they have any effect, it is secondary to cues acquired from comb during development.
Downs et al. (2000) investigated the effect that floral oils (anethole, citronellal, limonene and linalool) have on the probability of nestmates and non-nestmates being accepted by guard bees at nest entrances. Floral oils did not affect the probability of workers, either nestmates or non-nestmates, being accepted by guards. However, the presence of floral oils did increase the time taken for a guard to reject an introduced bee. These data show that guards are sensitive to floral oils but use other recognition cues when assessing colony affiliation.
Honey bees have the ability to distinguish among groups of larvae that are destined to become queens and preferentially rear highly related nestmate larvae over less related larvae that are not nestmates (Page and Erickson, 1984).
Colonies of honey bees from two patrilines (cordovan and dark) were established and observations were made on the behavior shown by the worker bees in rearing queen larvae within their colonies. The relationship among the bees within these colonies was either r = ¾ (super-sisters) or r = ¼ (half sisters). The worker bees showed preferential care to the queen larvae that were of their own patriline. Workers of the cordovan patriline showed a stronger preference for larvae of their own patriline than did the dark workers. Cordovan workers also showed a higher rate of visitation, indicating behavioral differences between the patrilines. These results suggest that kin selection is operating on honey bee behavior used in rearing reproduction (Noonan, 1986).
A honey bee queen is usually attacked if she is placed among the workers of a colony other than her own. This rejection occurs even if environmental sources of odor, such as food, water and genetic origin of the workers, are kept constant in laboratory conditions. The genetic similarity of queens determines how similar their recognition characteristics are; inbred sister queens were accepted in 35% of exchanges, outbred sister queens in 12% and non-sister queens in 0%. Carbon dioxide narcosis (stuper, unconsciousness) results in worker honey bees accepting non-nestmate queens. A learning curve is presented, showing the time after narcosis required by workers to learn to recognize a new queen. In contrast, workers transfer results in only a small percentage of the workers being rejected. The reason for the difference between queens and workers may be because of worker and queen recognition cues having different sources (Breed, 1981).
Boch and Morse (1974, 1979) have shown that honey bee queens can be recognized individually by swarms of bees. They found that marking a queen with shellac-based paint to give her a distinctive odor resulted in workers later exhibiting a preference for any queen marked with that paint. However, their experiments do not show whether the odors used by workers to recognize queens are produced by the queens or are environmentally acquired. In a series of studies concerned with queen introduction into colonies, Szabo (1974, 1977) also found that workers could discriminate among queens, but did not approach the issue of the source of recognition odors directly. It was also found that factors such as the age and weight of an introduced queen could affect worker choice among introduced queens. Yadava and Smith (1971) found that the mandibular gland contents of the queen were important in the release of worker aggression towards an introduced queen (Breed, 1981).
References
Boch, R. and R.A. Morse 1974. Discrimination of familiar and foreign queens by honey bee swarms. Ann. Entomol. Soc. Am. 67: 709-711.
Boch, R. and R.A. Morse 1979. Individual recognition of queens by honey bee swarms. Ann. Entomol. Soc. Am. 72: 51-53.
Bowden, R.M., S. Williamson and M.D. Breed 1998. Floral oils: their effect on nestmate recognition in the honey bee, Apis mellifera. Insectes Soc. 45: 209-214.
Breed, M.D. 1981. Individual recognition and learning of queen odors by worker honey bees. Proc. Nat. Acad. Sci. USA. 78: 2635-2637.
Breed, M.D. 1983. Nestmate recognition in honey bees. Anim. Behav. 31: 86-91.
Breed, M.D. 1998. Recognition pheromones of the honey bee. Bioscience 48: 463-470.
Breed, M.D. and T.M. Stiller 1992. Honey bee, Apis mellifera, nestmate discrimination: hydrocarbon effects and the evolutionary implications of comb choice. Anim. Behav. 43: 875-883.
Breed, M.D., M.F. Garry, A.N. Pearce, B.E. Hibbard, L.B. Biostad and R.E. Page, Jr. 1995. The role of wax comb in honey bee nestmate recognition. Anim. Behav. 50: 489-496.
Breed, M.D., E.A. Leger, A.N. Pearce, and Y.J. Wang 1998. Comb wax effects on the ontogeny of honey bee nestmate recognition. Anim. Behav. 55:13-20.
Dani, F.R., G.R. Jones, S. Corsi, R. Beard, D. Pradella and S. Turillazzi 2005. Nestmate recognition cues in the honey bee: differential importance of cuticular alkanes and alkenes. Chem. Senses 30: 477-489.
Downs, S.G. and F.L.W. Ratnieks 1999. Recognition of conspecifics by honey bee guards (Apis mellifera) uses non-heritable cues applied to the adult stage. Anim. Behav. 58: 643-648.
Downs, S.G., F.L.W. Ratnieks, S.L. Jefferies, and H.E. Rigby 2000. The role of floral oils in the nestmate recognition system of honey bees (Apis mellifera L.). Apidologie 31: 357-365.
Getz, W.M. and K.B. Smith 1983. Genetic kin recognition: honey bees discriminate between full and half sisters. Nature 302: 147-148.
Kalmus, H. and C.R. Ribbands 1952. The origin of the odours by which honey bees distinguish their companions. Proc. R. Soc. Lond. B. 140: 50-59.
Mann, C.A. and M.D. Breed 1997. Olfaction in guard honey bee responses to non-nestmates. Ann. Entomol. Soc. Am. 90: 844-847.
Martin, S.J., M.E. Correia-Oliveira, S. Shemilt, and F.P. Drijfhout 2018. Is the salivary gland associated with the honey bee recognition compounds in worker honey bees (Apis mellifera)? J. Chem. Ecol. 44: 650-657.
Noonan, K.C. 1986. Recognition of queen larvae by worker honey bees (Apis mellifera). Ethology 73: 295-306.
Page, R.E. Jr. and E.H. Erickson Jr. 1984. Selective rearing of queens by worker honey bees: kin or nestmate recognition. Ann. Entomol. Soc. Am. 77: 578-580.
Page, R.E. Jr., R.A. Metcalf, R.I. Metcalf, E.H. Erickson Jr. and R.L. Lampman 1991. Extractable hydrocarbons and kin recognition in honey bee (Apis mellifera L.). J. Chem. Ecol. 17: 745-756.
Szabo, T.I. 1974. Behavioural studies of queen introduction in the honey bee 2. Effect of age and storage conditions of virgin queens on their attractiveness to workers. J. Apic. Res. 13: 127-135.
Szabo, T.I. 1977. Behavioural studies of queen introduction in the honey bee 6. Multiple queen introduction. J. Apic. Res. 16: 65-83.
Yadava, R.R.S. and M.V. Smith 1971. Aggressive behavior of Apis mellifera L. workers towards introduced queens II. Role of mandibular gland contents of the queen in releasing aggressive behavior. Cand. J. Zool. 49: 1179-1183.
Clarence Collison is an Emeritus Professor of Entomology and Department Head Emeritus of Entomology and Plant Pathology at Mississippi State University, Mississippi State, MS.
]]>
The BIP team takes a break from inspecting colonies. From left to right: Eric Malcolm, Ben Sallmann, Cade Houston, Anne Marie Fauvel. © 2022, Eric Malcolm, beeinformed.org
 Looking for something to do Thursday, Sept. 14? Want to support BIP?
Looking for something to do Thursday, Sept. 14? Want to support BIP?
Join BIP LIVE for an hour of enjoyable conversation with beekeeping YouTuber David Burns! He, alongside Bee Informed Partnership’s Anne Marie Fauvel and Eric Malcolm, will discuss what BIP is all about and chat about honey bee health.
David is sponsoring this livestream fundraising event as an opportunity to share more about BIP’s work with the community and help raise much-needed funds to support their mission.
Stop by for a visit and stay for the fun by visiting David Burns’ YouTube channel on Thursday, September 14 at 8pm EST / 7pm CST!
YouTube link not working? Copy and paste this link into your browser’s search bar instead: https://www.youtube.com/watch?v=8nCvJNSE_JY
Did you know David Burns writes an article in Bee Culture every month? Check out his article from June and then subscribe to never miss one of his article! June article: https://www.beeculture.com/hot-hive-inspections/
]]> By: Tina Sebestyen
By: Tina Sebestyen
The bottom line answer to the question, “Why do beekeepers give up?” is: because their bees die, usually over and over. They discover how hard it is to keep bees alive. Someone in my beginning beekeeping class always pipes up to ask why I say that it is hard to keep bees alive, when they live just fine all alone, on their own, out in the woods. That is not the only misconception new beeks start out with. Unfortunately, bees do not live just fine on their own without human help. In fact, they are never on their own, they are sickened and harmed by Varroa destructor constantly and thus 97.5% of colonies cannot live long without human help to control this honey bee predator.
It follows that new beekeepers who think that bees live fine on their own also don’t have any idea how much time they are going to need to take care of their bees. They don’t spend enough time getting comfortable handling the bees, so they’re unable to see and recognize when there is a problem. They aren’t doing good inspections. One of the leading indicators of bee survival is beekeeper experience. Every new beekeeper starts out with no experience, but getting in that hive every week and exploring, observing and manipulating (mite counts, anyone?) are where one can gain that valuable experience.
Once a beekeeper gets over their romanticized idea of beekeeping, decides to get serious and puts on their big kid pants, they often go to the internet to learn how to keep bees. Don’t get me wrong, not everything on the internet is bad or wrong… neither is it all good or right, and new beekeepers have no way of knowing which is which. Even if all of the information found on YouTube was correct, so much of beekeeping is local. It is dictated by whether or not water falls from the sky (I hear that is called rain, we don’t know it here in CO), temperature swings, timing and bounty of forage, the presence of small hive beetles and so many other things.
One of the things new beekeepers are looking for online is a recipe for beekeeping. Something like: feed until May 15th, add boxes on June 12th, split on X date. Unfortunately, beekeeping is not like baking cookies. You need to be able to observe what is happening, extrapolate what is about to happen and come up with a plan to help the bees do what they want to do. It also is not like owning a puppy or kitten, which you can run to the vet if something mysterious happens to their health.
Beekeeping is a lot more like owning livestock, maybe a herd of cattle. As such, their health needs to be safe-guarded in advance of trouble. They need water, adequate and varied forage, and protection from wind and predators. After trouble comes, it is harder to rectify than it would be with a pet.

One great reason to join a bee club is that most have teaching apiaries. This time, we were having a potluck, and our host allowed us to go play with his bees. We often seek volunteers for apiary visits, which allows us to see surprising things, and gives a variety of situations to discuss, besides planned events at club hives.
Happily, there are solutions to these situations. One of the best, most fun, and most fruitful is to join a local bee club. Here you will find beekeepers who know what works in your area, starting with what sub-species of bee is right for you. These seasoned beeks love to share what they’ve learned the hard way, if you can just be humble enough to take them seriously, instead of thinking you’ll be able to make what wouldn’t work for them be successful for you.
One reason people don’t take the advice they are given is because they don’t understand the bee biology and behavior that is the reason behind the management or the timing of the decision. Our best beekeepers are those who read a lot about beekeeping. And, there is a lot out there. It has been said that more has been written about beekeeping than anything else besides religion. Again, what you find on the internet may have been written by a (notoriously over-confident) third year beekeeper. Books that have been published on paper by a real publisher, rather than a vanity, do-it-yourself publisher, have gone through at least some kind of vetting process. Bee journals do great reviews of such books, to help you know what will be readable and what is worthwhile information. Those journals themselves are a great source of current, vetted, information.
New (and old) beekeepers need to learn bee math, bee biology and management. These topics sound like what you wanted to avoid in high school, but they are a lot more fun in the real world, with real application in beekeeping. Once you get started, you’ll fall in love even more deeply with beekeeping. There is so much to discover and learn still!
And now to the brass tacks… beekeepers give up because bees die, and we need to learn about why they die and help them manage their challenges in advance. Bees have trouble with the five Problems. Pests, Pathogens, Poor forage, Pesticides and Politics. Every beekeeper has chosen one P, THE one that they think is THE problem, that if we just solved that one problem, everything would be great. Paying attention to one Problem without watching out for the others is another recipe for dead bees.
The most famous of the Pests is Varroa destructor, and undoubtedly, if we could solve that one, beekeeping would be a whole different proposition. But even without varroa, bees still need weeds which are in short supply in our world today, and varied sources of nectar and pollen throughout the collecting year. And, they still need a clean world to live in, one not poisoned by Pesticides. They need pro-active maintenance to be strong enough to overcome the Pathogens: viruses, bacteria and fungi. And they need us to have laws that are beneficial to their world and their keeping.
To sum it all up, if you want to continue to keep bees and have them live, so that you don’t get so frustrated with them dying all the time that you quit, here is what you need to do.
- Join a bee club and attend meetings in person. Get a mentor if you can.
- Read a lot about beekeeping, about bee biology and behavior, from books and magazines.
- Do what your mentor or local bee club advises, even if you don’t understand why right now.
- Learn to do an effective hive inspection. Plan to spend at least one hour per week on a pair of hives (one hive alone is a recipe for doom) for your first year, and at least one hour every other week thereafter.
Good luck, and happy beekeeping!
]]>Click Here if you listened. We’re trying to gauge interest so only one question is required; however, there is a spot for feedback!
Read along below!
 My Apiary Ecosystem
My Apiary Ecosystem
Honey bees are only a part of it.
By: James E. Tew
There’s always going to be something
In my hives or in my life, there is always going to be something – some issue or some problem. I literally just finished a phone call with one of my adult daughters. She had just eliminated a harmless Wolf Spider (Tigrosa annexa) because it frightened her young son (my nine-year old grandson). A few weeks ago, she had a problem with ants in her kitchen, but now they are gone. Now, she has Springtails (Collembola) in one of her bath showers. She complained to me that she feels that her home is under constant attack. I tried to tell her that there is always going to be something going awry. Always. Chill out. I don’t think she listened to me, but I listened to her.
Since I have spent my adult life studying honey bees, she assumed that I was also an information resource for Springtails. Readers, I don’t know anything about these small flea-sized arthropods, but unintentionally, my daughter set me to thinking and exploring. What do I know about Springtails? In all my beekeeping years, I have never asked or thought about the presence of Collembola in bee hives.

Figure 1. Springtails are about the size of a flea and seemingly cause no harm to bees or beekeeping.
Springtails are common in organic materials that are being degraded. On a whim, I keyed in a web search on Springtails (Collembola) in bee hives. I immediately got hits. All the citations that I found were from observant beekeepers. Having not found any information from academic or regulatory sources, I went to an AI open-source app and was given the following, undocumented information.
Collembola, commonly known as Springtails, are small arthropods that belong to the class Collembola. They are found in a wide range of habitats, including soil, leaf litter and decaying organic matter. While they are not typically associated with bee hives, it is possible for Springtails to be present in bee hives under certain conditions.
Springtails are detritivores, meaning they feed on decaying organic matter and microorganisms. In a bee hive, there may be small amounts of organic debris, such as pollen, beeswax and other residue, which could provide a food source for Springtails. However, the presence of Springtails in a bee hive is usually considered incidental and not a significant problem for honey bees.
I had no idea
I had no idea that these small animals could occasionally be found in hives, but my conversation with my daughter came at a time that I had been pondering this very concept. What is routinely in my bee hives and in my apiary other than honey bees? I have never found a comprehensive listing of the lifeforms that I could expect to find there.

Figure 2. Fire ants and my honey bees. Do the ants help or hurt my bees? Ants, of many species, are common in my beeyard.
My apiary is essentially a distinct ecosystem. My apiary is where I keep my bees, but I have long since realized that many other species find that an apiary is home for them, too. These “unknowns” can apparently fall into three broad categories: harmful, beneficial or neutral.
The Big List
Early in our beekeeping journeys, we are exposed to what I have named the “Big List.” Some of the common entries on this list are raccoons, skunks, ants, wax moths, small hive beetles, birds, toads and mice. It is not my intent to discuss these common hive intruders here. Yet, in the hours and hours that I have either sat by my colonies or pawed through them, I routinely see other species and wonder what they’re up to as they bum around my hives. Usually, their presence remains a mystery.
Flies
Of course, there are numerous species of flies in and around my hives and colonies. We’ve all seen them. In fact, the classic book, Honey Bee Pests, Predators, & Diseases (Morse, Roger A. & Kim Flottum. Honey Bee pests, Predators, & Diseases. A.I.Root Company, Medina, Ohio. 44691. 718 pp. Chpt 8. P 143-162.) has a designated chapter on various fly species and their effects on bee colonies. Essentially, flies and their associated larvae are degraders and generally, do not have a harmful effect on healthy bees.

Figure 3. Common Green Bottle Fly (Blow Fly), probably Lucilia sericata, parked on the front of one of my active hives.
But, I can’t help but notice the occasional Green Bottle Fly nosing around my active hives. They are very flighty and will not allow a close camera shot before taking quick flight. Why are they there? I never see them get inside the hive.

Figure 4. Black Soldier Fly larvae, H. illucens larvae, on honey bee frame. (Scott Razee photo)
But there are unique encounters between various Dipterous species and honey bees that are rare. Anthony (Auth, C. Anthony, Hauser, M. & Hopkins, B.K. A scientific note on a black soldier fly (Stratiomyidae: Hermetia illucens) infestation within a western honey bee (Apis mellifera) colony. Apidologie 52, 576–579 (2021). https://doi.org/10.1007/s13592-021-00844-y) published a scientific note on a Black Soldier Fly (Stratiomyidae: Hermetia illucens) infestation within a western honey bee (Apis mellifera) colony. In the article abstract, Anthony, et al. reported:
Black soldier Fly larvae (Hermetia illucens) were discovered in a weak honey bee colony in Hailey, Idaho. The larvae were localized to the brood area and caused the affected comb to putrefy. Further communication with the beekeeper revealed that the colony recently returned from California and that the larvae likely originated there as well. In California, H. illucens are common and exist sympatrically with honey bees, yet there have been very few reports of damage. We therefore believe H. illucens are unlikely to cause damage to healthy colonies or significantly impact the apiculture industry. This report is the first published observation of H. illucens in Idaho and shows conclusively for the first time that H. illucens associates with honey bee colonies in North America.

Figure 5. Adult Black Soldier Fly (Utah State University Extension)
There are distinct Diptera species that either are outright harmful to bees or other flies that are considered to be minor pests. Examples of damaging species are Phorid Flies (Zombie Flies) while a lesser pest is the bee louse (Braula caeca). These pests are documented elsewhere in the beekeeping literature. I will not cover them here.
Earwigs

Figure 6. Adult European earwigs on the inner cover of my hive.
How bad can earwigs be? In Alabama, earwigs are commonly found in honey bee colonies in significant numbers. No doubt they are found in other states. I have noted that earwigs were not a common hive resident in Ohio, but that all changed a few years ago. I now have earwigs in my office, in my home and in my bee hives.
I don’t know that they do anything within the hive. I faintly remember one Russian paper, that reported that earwigs could be alternate hosts for the bacteria causing European Foulbrood. Other than that one point, I have never heard complaints about them.
Of course, they are a pain when extracting and must be caught in the filter when processing honey. Straining bees and bee parts from honey is bad enough, but earwigs in the honey filter are unsightly. I don’t know of anything that you can do about them. In fact, I’m not sure anything should be done about them.
Roaches
As an entomologist, I respect roaches. They are the consummate survivor. Obnoxious that they are to humans, one must respect their persistence. Many, many developmental years ago, roaches “learned” to fold their wings over their bodies so they could exploit more niches, than say, a broad-winged insect like a dragonfly (dragonflies will also occasionally prey on honey bees). By storing their folded wings over their bodies, cockroaches could, more easily, get under your refrigerator or inside your bee hives. They have been a challenge for me everywhere I have kept bees.
Like so many other insect visitors/invaders, roaches are drawn to both the sweet food supply as well as the protein supplies – and then there are all the decaying larvae and adult bees to munch on. The question is begged, “Why would roaches NOT be in our hives and stored equipment?”
In the beekeeping literature, it is often written that the damage roaches do is minimal, but I want to loudly say that the appearance of just a single roach in your honey house can dissuade even the staunchest customer who is considering buying your honey. And then there is the excreta to consider. In fact, one of my most serious concerns of both cockroaches and earwigs is the excrement that they leave behind.

Figure 7. Unfortunately, a cockroach is not an uncommon hive visitor.
It is also commonly written that cockroaches are primarily found in weakened or otherwise ailing colonies. Yes, that is surely true, but I will loudly say that great numbers of roaches happily live on the inner cover of populous colonies. When the outer cover is removed, in a flash, they scamper down into the bees.
In my opinion, there is little to be done to control a roach infestation. Protecting the customer and protecting the purity of the honey product is about all that can be done. Yet, I cannot conclusively say that a modest roach infestation is absolutely harmful to the bees. Maybe future studies will continue to add information to this colony co-habitant.
Honey Bee Cousins

Figure 8. A yellowjacket lunching on one of my dead honey bees.
Yellowjackets
Though yellowjackets are on my “Big List” and are common pests in the beeyard, I have included them here. In my yards, these brightly colored insects could almost be a traditional resident of my apiary. Yellowjackets get included on my “mystery” list due to technicalities.
Yellowjackets (probably Vespula maculifrons) readily see their honey bee cousins as a potential food supply. On occasion, these wasps may have a nest in my apiary, but much more likely is that these hymenopterous insects are simply foraging within my apiary.
Experienced beekeepers have commonly seen yellowjackets mulling around the detritus at the hive entrance, but they will also enter a weakened colony and take honey, pollen, brood and adults depending on their own colony needs.
As such, yellowjackets get an entry on the common list of bee colony pests, but they do not seem to be the effective cause of the colony’s decline but are more commonly responding to a colony that has been weakened by other factors. Yellowjackets can be numerous when robbing behavior is rampant.

Figure 9. Bumblebees can frequently be seen attempting to enter a honey bee colony. This can be disastrous for the bumblebee with deadly results.
Bumblebees
I frequently see bumblebees trying to enter a bee colony. How suicidal is that? Only on a few occasions have I stumbled onto a bumble nest in unused honey bee equipment, but they are frequently in my apiary surely searching for food.
In fact, bumblebees may not necessarily be attracted to my honey bee colonies specifically, but they may be drawn to certain characteristics or resources associated with honey bee colonies. I can come up with a few reasons that may explain why bumblebees are sometimes found near my honey bee colonies:
- Floral resources: Honey bee colonies are known to forage on a wide variety of flowering plants to collect nectar and pollen. Bumblebees, like honey bees, are also generalist foragers and seek out similar floral resources.
- Odor cues: Honey bee colonies emit a combination of pheromones and odors that can be detected by other bees, including bumblebees. These chemical signals may serve as attractants, potentially drawing bumblebees to the entrance of the honey bee colony.
- Nesting opportunities: While honey bees typically nest in enclosed structures such as beehives or tree cavities, bumblebees often create nests in the ground or other protected locations. However, bumblebees may occasionally take advantage of abandoned honey bee hives or other suitable cavities near a honey bee colony, which could lead to their presence in the area.

Figure 10. The apiary ecosystem can become complicated. This mantis is eating a yellowjacket that it caught while sitting atop one of my hives.
It’s important to note that interactions between bumblebees and honey bees can vary depending on the specific circumstances and the behavior of individual bees. While they may coexist peacefully in some cases, competition for limited resources, like nectar or nesting sites, can also occur.
My core point for this article
The apiary is an active place, but not only for honey bees. Many other common and not-so-common insects and animals hang around the beeyard foraging for food or shelter. You and I take a staunch view of these unwanted visitors. But truthfully, we do not know a lot about the complex interaction between all these other species and our honey bees.
For instance, Caron (Caron, Dewey. In Honey Bee Pest, Predators, and Diseases cited elsewhere in this article.), listed more than fifteen different beetle species that could be found in beehives – usually in the bottom board litter. Most beetle species had no ill effect on our bees, but I can’t help but wonder if some of those species could be beneficial to our honey bees. Currently, no one seems to know.
In this article, I have only tinkered with a few of the species that we can see with our unaided eyes. I made no effort to review the macroscopic visitors. Examples are: protozoans, fungi and nematodes. They’re all there, too. Readers, I sense that I’m describing a bee hive supermarket or maybe a big box store for insects.
Not wanting to run amok here, but it appears to me that the apiary is a significant food and shelter resource for a lot of other insects and animals. As beekeepers, we laud the effectiveness of the workers’ defensive stinger, but this protective device does not seem to be very effective against spiders, earwigs, birds or a fungus particle. I’m guessing that out of simple necessity, bees coexist with many of these interlopers. Hive visitors come in differing sizes and represent many diverse species.
Despite everything the bees and their keepers can do, the apiary will seemingly continue to be an attractive site for non-bee species. In this article, I’m trying to ask, “From a diversified ecological position, is this necessarily a bad thing?” Consider how many other species are being subsidized and nurtured by resources from our apiaries. Could it be said that our bee hives are “community centers” for a host of non-apis species? I think so. Blame my daughter. She started this.
Thank you for reading and thinking.
Dr. James E. Tew
Emeritus Faculty, Entomology
The Ohio State University
[email protected]
 Co-Host, Honey Bee
Co-Host, Honey Bee
Obscura Podcast
www.honeybeeobscura.com
Click Here if you listened. We’re trying to gauge interest so only one question is required; however, there is a spot for feedback!
Read along below!

Pure Honey?
Probably Not So Much
By: Ross Conrad
Most widely known as components of Teflon coated cookware and firefighting foams, PFAS are extremely persistent in the environment. Researchers don’t really have a very good handle on how long it takes for them to actually break down, if they ever do, which is why they are sometimes referred to as forever chemicals.
PFAS Everywhere
Due to their unique properties, these chemicals are used in an extremely wide range of commercial, industrial and consumer products, including adhesives, building and construction materials, cleaning products, paints, varnishes and inks, cosmetics and personal care, dry cleaning, flea and tick products, electronics, explosives and ammunitions, the oil and gas industries, and the medical industry, among others (Gaines, 2021). A huge number of PFAS chemicals have been produced and distributed through the global supply chain, with over 9,000 PFAS related chemicals recorded dating back to before 1950 according to the Environmental Protection Agency Master list of PFAS substances (EPA).
High Level of Toxicity
Peer reviewed studies have connected exposure to certain PFAS chemicals to a host of human health problems including reproductive effects such as decreased fertility or increased high blood pressure in pregnant women, developmental effects or delays in children, increased risk of some cancers, suppression of the body’s immune system and interference with the body’s natural hormones, along with increased cholesterol levels and/or risk of obesity (EPA, 2023). Some of these chemicals are so toxic that in 2022, EPA released a lifetime drinking water health advisory for four PFAS substances – not in the amounts of parts per million, or parts per billion, but in the fractions of parts per trillion range (U.S. Gov., 2022).
Health effects associated with exposure to PFAS are difficult to nail down for many reasons. Although there are thousands of PFAS with potentially varying effects and toxicity levels, most studies have focused on a limited number of better known PFAS compounds. Meanwhile, people can be exposed to PFAS in different ways and at different stages of their life, and the types and uses of PFAS change over time. All of this makes it challenging to track and assess how exposure to these chemicals occurs and how they will affect human health.
Honey Bee Exposure
In the June issue of Bee Culture, I note that PFAS used in plastic manufacturing (most notably in high density and low density polyethylene – HDPE & LDPE) has the potential to leach out of plastic hive components and impact bees and honey. Well, it turns out there is yet another avenue for PFAS to potentially make its way into our hives: pesticides (Lasee et al., 2022).
PFAS chemicals are being found in a wide variety of pesticides. Sometimes, the PFAS is the active ingredient or added as an adjuvant, or so called “inert” ingredient included in the pesticide formulation to enhance the effectiveness of the active ingredient. Other times, the levels of PFAS found in a particular pesticide is so small it is likely a result of the chemical leaching out of plastic packaging and into the pesticide or its components prior or during manufacture. Since pesticides are applied to our food crops, researchers are documenting PFAS chemical build up (bioaccumulation) in fish, animals and people.
Officials in the state of Maine found that more than 1,400 pesticides contain active ingredients that meet the state’s definition of PFAS (EWG, 2023). Researchers have also documented the migration of toxic pesticides from the surrounding environment into honey bee colonies.
The common presence of PFAS in pesticides, potentially including those many beekeepers place in their hives, is concerning not just from a human perspective but also from the bee’s. A small but growing body of research reveals the potential for adverse impacts of PFAS exposure in honey bee colonies. One researcher found that a honey bee’s oral exposure to PFOS resulted in a 72-hour oral median lethal dose (LD50) of 0.40 mg per bee (Wilkins, 2001). Meanwhile, a correlation between the bioaccumulation of fluorinated pesticides in honey bees, along with other types of pesticides, and mass mortality events of honey bee colonies has been documented (Martinello et al., 2019). Another study found that not only do PFAS have the potential to adversely impact honey bees, they can migrate into honey, the primary source of carbohydrate and energy for honey bees and one of the primary hive products produced by beekeepers (Sonter et al., 2021). Given the well-established fact that pesticides are commonly found in honey samples, it should come as no surprise if further testing uncovers widespread PFAS contamination in honey sold for human consumption.
States Taking the Lead
PFAS contamination presents a mostly unexamined problem for farmers all across the country. Prior to the recent revelation of PFAS contamination of pesticides, farmland has historically been contaminated with PFAS through the spreading of waste water treatment plant sewage sludge as an agricultural fertilizer. Another acknowledged source of contamination is the leaching of PFAS chemicals off military bases onto nearby farms and water supplies.
As part of the effort to get a handle on this situation, Maine has become the first state to enact a comprehensive ban on pesticides that include intentionally added PFAS, as well as pesticides contaminated with PFAS. The ban is currently set to take effect in 2030.
A second state to move to ban PFAS is Minnesota. Scientist’s understanding and ability to detect PFAS in the environment has evolved greatly since the Minnesota Pollution Control Agency (MPCA) and the Minnesota Department of Health (MDH) began investigating them back in 2002. Laboratories at that time had only identified two PFAS and extremely low concentrations were undetectable given the existing technology at that time. Today, we are able to measure extremely small amounts (parts per trillion) of several PFAS. As noted before, studies have linked long-term exposure to PFAS in this range to negative outcomes especially for the most vulnerable members of our population: children, the elderly, pregnant women and those with compromised immune systems.
The writing is on the wall when it comes to PFAS compounds. One of the world’s primary PFAS manufacturing companies, 3M, has agreed to pay more than $10 billion to settle lawsuits claiming it knowingly used “forever chemicals” in its products despite being aware of risks to human health. Additionally, 3M says it’s phasing out two common compounds – PFOS and PFOA – and has announced that they will discontinue all types of the chemicals by 2026. Unfortunately, history has shown that companies usually only phase out a toxic compound when they have a replacement ready for market. Typically, the replacement is just as harmful as the compound it replaces, but it takes decades to accumulate the data showing harm and require the replacement chemical to also be withdrawn. Of course by then, yet another toxic replacement is found.

Regularly rotating old comb out of hives is one way beekeepers can reduce toxic chemical loads on bee colonies. The color of the comb is not a reliable indication of the age of the comb, since combs that are only used by the bees or food storage take much longer than brood combs to darken.
Varroa Treatments
The commonly used beekeeping pesticide Amitraz (sold as Apivar) was not found to contain any fluorinated chemicals that would meet the state of Maine’s definition of PFAS. However, Fluvalinate, the active ingredient in Apistan does meet the state’s definition.
I spoke with pesticide toxicologist, Pam Bryer of the Maine Board of Pesticide Control who pointed out that the PFAS problem could easily have been avoided if all chemicals were treated like pesticides which are regularly tested for their persistence in the environment. According to Bryer, “for most of the PFAS out there, there is almost no data.” Bryer assured me that unlike PFOS and PFOA which are long chain fluorinated compounds, fluvalinate is not nearly as toxic since it contains a short chain fluorinated methyl group. She did express concern, however what can happen should the fluorinated methyl group in fluvalinate combine with other chemicals potentially forming more toxic compounds or potent green-house gases.
Better Safe Than Sorry
In my mind, the safest treatments available to beekeepers today are those that utilize organic acids. By definition, the acids are not toxic, though they are corrosive. All of the organic acids (formic, oxalic and hop beta) that are approved for use to control varroa mites become neutralized over time and leave behind no harmful residue from the acid. Unfortunately, we now know that PFAS have the potential to make their way into such otherwise relatively safe products and contaminate our colonies despite our best efforts.
Beekeepers don’t have to wait for regulatory action and should consider increasing their reliance on natural and organic approaches, or when possible, treatment-free management techniques to control mites. As beekeepers, we can reduce or even eliminate toxic chemical use in our hives starting today by incorporating organic practices into our hive management. One way is to regularly rotate old combs out of hives and allow colonies to build new comb so the level of residue buildup in wax remains relatively low. Another approach discussed in my book, Natural Beekeeping, includes the use of screened bottom boards, culling capped drone brood, forcing a brood break in the hive, and propagating strains of bees that show some resistance to mites and diseases. A trial that ran between 2016-2019 found that combining all five of these physical and biological treatment-free management approaches can control mite-related hive mortality and ensure survivability on par with commercially available pesticide treatments (Conrad, 2021).
Recently, researchers Robyn Underwood, Margarita López-Uribe and their team from Penn State and Virginia Tech published a study on organic beekeeping in which they “… found that the organic management system-which uses organic-approved chemicals for mite control-supports healthy and productive colonies, and can be incorporated as a sustainable approach for stationary honey-producing beekeeping operations.” (Underwood et al., 2023).
As Rachel Carson noted over 60 years ago: “The most alarming of all man’s assaults upon the environment is the contamination of air, earth, rivers and sea with dangerous and even lethal, materials. This pollution is for the most part irrecoverable; the chain of evil it initiates not only in the world that must support life, but in living tissues is, for the most part, irreversible.” (Carson, 1962). Given that we now have effective low toxic and non-toxic alternatives to dangerous chemicals, why continue to play Russian roulette with pesticides?
Ross Conrad is author of Natural Beekeeping, Revised and Expanded 2nd edition, and co-author of The Land of Milk and Honey: A history of beekeeping in Vermont.
References:
Carson, Rachel (1962) Silent Spring, The Riverside Press Cambridge, Houghton Mifflin Company, Boston pg 6
Conrad, Ross (2021) Comparison of a commercial Varroa mite honey bee treatment with treatment-free Varroa management techniques, Bee Culture September: pp 41-45
Environmental Protection Agency, Comptox Chemicals Dashboard: Master List of PFAS Substances (Version 2). Accessed June 26, 2023. https://comptox.epa.gov/dashboard/chemical_lists/pfasmaster
Environmental Protection Agency (2023) Our current understanding of the human health and environmental risks of PFAS, accessed June 21, 2023 https://www.epa.gov/pfas/our-current-understanding-human-health-and-environmental-risks-pfas
Environmental Working Group (EWG), Maine data unveils troubling trend: 55 PFAS-related chemicals in over 1400 pesticides, accessed June 13, 2023 – https://www.ewg.org/news-insights/news-release/2023/06/maine-data-unveils-troubling-trend-55-pfas-related-chemicals
Gaines, Linda G.T. (2001) Historical and current usage of per- and polyfluoralkyl substances (PFAS): A literature review, American Journal of Industrial Medicine, 66:353-378
Lasee, S. et. al. (2022) Targeted analysis and total oxidizable precursor assay of several insecticides for PFAS, Journal of Hazardous Materials Letters, 3:100067
Martinello, M. et. al. (2019) A survey from 2015 to 2019 to investigate the occurance of pesticide residues in dead honey bees and other matrices related to honey bee mortality incidents in Italy, Diversity, 12(1):15
McCarthy, C., Kappleman, W. & DiGuiseppi, W. (2017) Ecological Considerations of Per- and Polyfluoroalkyl Substances (PFAS). Curr Pollution Rep 3, 289–301
Steven Lasee, Kaylin McDermett, Naveen Kumar, Jennifer Guelfo, Paxton Payton, Zhao Yang, Todd A. Anderson, (2022) Targeted analysis and Total Oxidizable Precursor assay of several insecticides for PFAS, Journal of Hazardous Materials Letters, Vol. 3.
Sonter, C., Rader R., Stevenson, G., Stavert, J., Wilson, S.C. (2021) Biological and behavioural responses of European honey bee (Apis mellifera) colonies to perfluorooctane sulfonate (PFOS) exposure, Integrated Environmental Assessment and Management, 17(2)
United States Government (2022) Lifetime Drinking Water Health Advisories for Four Perfluoroalkyl Substances [FRL 9855-01-OW], Federal Register 87:118 pp 36848-36849
Underwood, R.M., Lawrence, B.L., Turley, N.E., Cambron-Kopco, L.D., Kietzman, P.M., Traver, B.E., López-Uribe, M.M. (2023) A longitudinal experiment demonstrates that honey bee colonies managed organically are as healthy and productive as those managed conventionally, Scientific Reports, 13:6072 https://doi.org/10.1038/s41598-023-32824-w
Wilkins, P. (2001) “Perfluorooctanesulfonate, potassium salt (PFOS): An acute contact toxicity study with the honey bee. Study number HT5601.”
Click Here if you listened. We’re trying to gauge interest so only one question is required; however, there is a spot for feedback!
Read along below!
Found in Translation
Sweet and Sour Honey
By: Jay Evans, USDA Beltsville Bee Lab
There are many ways that honey bees improve our diets but honey consumption was an early reason to wrangle this species. The taste for honey persists today around the world, sustaining sideliners, families and large corporations in many parts of the world. It is also widely known to soothe and improve relations with neighbors, in-laws and bosses. With any high-value product, there is a risk of inadvertent or purposeful false advertising.
One honey quality trait that is easy to control is water content. Small-scale beekeepers routinely put their honey crops and relationships at risk by bottling honey that hasn’t been fully processed by bees to a net water percentage under 19%. Watery honey both feels weird and is prone to unintended fermentation. Choosing properly capped frames goes a long way to eliminating this problem. If you live in a humid place like Maryland, there is also some risk that open honey will dehumidify some of the local air, pushing water content back above dangerous levels. Truly dry honey can be achieved by technique and awareness, but if you are curious and want to directly assess the water content of your crop, Hanna Bäckmo gives a nice review of the styles and costs of refractometers used by beekeepers in this magazine (https://www.beeculture.com/refractometer/). Certainly, steady honey producers would benefit from investing in, and calibrating, these things.
A bit out of reach for most of us, but essential for the industry, are lab-based assays aimed at confirming honey purity. The methods used for this continue to improve, putting clumsy or sneaky honey producers on notice. Notably, honey yields can be stretched by a variety of refined or expelled sugars. This might be inadvertent, when syrup fed by beekeepers in the Fall for Winter survival lingers, capped until Spring. There is no easy answer to this, certainly not from me, but step one is to get bees through Winter safely, and then assess any remaining capped stores to see if these stores are bona fide honey or syrup that bees dried down but didn’t gobble up as it came in. Ask a beekeeper near you for help.

Photo by Meggyn Pomerleau on Unsplash
More insidiously, producers or packers might outright add less expensive fillers to their honey, increasing yields but losing some of the magic of honey. The technology used to detect such adulteration is improving, and several techniques are now used by regulators, producers and packers to make sure honey is pure. The International Honey Commission described forensic methods for honey purity nearly 30 years ago and updated these methods in 2009 (https://www.bee-hexagon.net/english/network/publications-by-the-ihc/). The U.S. Food and Drug Administration, keeping honest folks honest across the industry, regularly tests new methods against imported and domestic honey to identify so-called ‘economically motivated adulteration’. Using a well-established technique, Stable Carbon Isotope Ratio Analysis (SCIRA), the FDA recently screened bulk and bottled honey samples from eight countries whose honey is imported into the U.S. (https://www.fda.gov/food/economically-motivated-adulteration-food-fraud/fy2122-sample-collection-and-analysis-imported-honey-economically-motivated-adulteration). This test distinguishes ‘C4’ plant sources (largely grasses and grains) from ‘C3’ sources (all the plants with prettier, bee-visited, nectar-rich flowers). The test simply asks if the unexpected C4-sugars, often from corn syrup or sugar cane, are over-represented in honey. There is some tolerance of these C4 sugars due to bee management or assay imprecision but that level is quite low, maybe 7% by volume. Each country in the FDA screen had at least one suspicious honey batch, but the overall frequency of such batches was 10%, a level roughly similar to a much larger recent study in Europe and indicative that honey, by and large, is as advertised.
There are several newer techniques in play now for the high-stakes race between regulators and those who might diminish the reputation of honey. Dilpreet Singh Brar and colleagues in A comprehensive review on unethical honey: Validation by emerging techniques (Food Control 2023, 145, 109482, https://doi.org/10.1016/j.foodcont.2022.109482) describe nearly 50 ways to test your clover. Within the alphabet soup of available methods, they reveal six chromatographic platforms (basically methods to separate parts of a whole by size, electric charge or affinity to some sort of ‘bait’) with increasing sophistication. These machines should put fear in anyone whose honey is not perfectly sound.
As a geneticist, I am fascinated with so-called environmental DNA (eDNA) screens, whereby a complex soup is scrutinized for the genomes of the diverse organisms floating in it. Many will remember the application of eDNA screens worldwide to identify levels and variants of the SARS-Cov-2 virus in city and town wastewater systems (poor interns!; https://www.nih.gov/news-events/nih-research-matters/tracking-sars-cov-2-variants-wastewater). This same methodology is now widely used to confirm the botanical sources of honey, the genotypes of the bees collecting that honey and the myriad of other organisms from the hive environment. Practically, this method also precisely identifies any honey contaminant with a biological source, from corn syrup to diverse flower sources mixed in accidentally in coveted monofloral honeys. It is also a sensitive assay for honey bee disease agents.
For the past 20 years, genetic analyses of honey from hives have been used to confirm the presence of the bacterium responsible for American Foulbrood, Paenibacillus larvae. Federico Lauro and colleagues in Rapid detection of Paenibacillus larvae from honey and hive samples with a novel nested PCR protocol (International Journal of Food Microbiology 2003, 81, 195-201, https://doi.org/10.1016/S0168-1605(02)00257-X) showed the value of this technique for keeping track of non-symptomatic P. larvae populations. More broadly, Leigh Boardman and others have confirmed that this technique can provide a snapshot of the whole range of microbes found in colonies (Boardman, L., P. Marcelino, J. A., Valentin, R. E., Boncristiani, H., Standley, J. M., & Ellis, J. D. Novel eDNA approaches to monitor Western honey bee (Apis mellifera L.) microbial and arthropod communities. Environmental DNA. 2023; https://doi.org/10.1002/edn3.419). Here, colony-collected honey is analogous to the worker-bee samples now used in many disease surveys. Honey collections have the added value of pointing out long-ago arrivals, providing a sort of fossil record for the plants and other organisms a colony might have come into contact with during the past year. The genetic methods behind these screens are astoundingly sensitive (remember, viruses floating alone in tons of sewer sludge) and honey or hive-based screens have promise for anything from virus outbreaks to the detection of newly invasive mites and other pests. It is incredibly hard to pass through an environment without shedding a little DNA, and a little goes a long way for these sensitive methods.
Economically motivated adulteration is detectable with some effort and that’s a good thing for all of us. Honey screening, especially with the twist of identifying genetic signals from hive organisms, is also becoming a nice tool for scientists keen on monitoring disease, plant sources and the genes of the bees that did all the work.
]]>Instead of writing about a pollinator plant, I want to address the weary accusation that somehow honey bees outcompete native bees and “don’t belong here” or “we can do just fine without honey bees.” Lately this controversy has gained steam despite countless research articles showing that honey bees and native bees have been getting along just fine for centuries, thank you. Truthfully, these pollinators supplement each other on our behalf. A study by Greenleaf and Kremen observed that interactions between wild bees and honey bees doubled pollination rates and enhanced the prevalence of hybrid sunflowers by five-fold.
Honey bees have been in the United States since at least 1622, but most likely since the 1500’s when Spaniards established St. Augustine, Florida – USA’s oldest city – to obtain beeswax for their candles. Recent research discovered a fossil of the honey bees’ relative Apis neararctica spp., in Stewart Valley, Nevada that dated back to the Miocene Era. So, the honey bees have been getting along with native bees for centuries!
So, what has changed? Mankind, or not-so-kind. As areas are bulldozed and stripped of woods, fields, wetlands and streams, the insects, birds and other wildlife lose places to nest, find food and shelter and exist. Pristine lawns and tidy shrubs do not provide habitat for most pollinators, let alone other wildlife. 50 million acres of perfect suburban lawns in the U.S. doesn’t help. An environmental ‘black hole’.
Honey bees are generalists with short tongues, meaning that they prefer flowers with shallow centers so that they can access the pollen and nectar easily. They like the flowers of many trees, shrubs, perennials, bulbs and annuals, including some non-native “weeds” like Japanese honeysuckle, Autumn olive and spotted knapweed. Generally, they are active on sunny days when the temperature is over 60°F, and back in the colony by evening to cool or warm the colony.
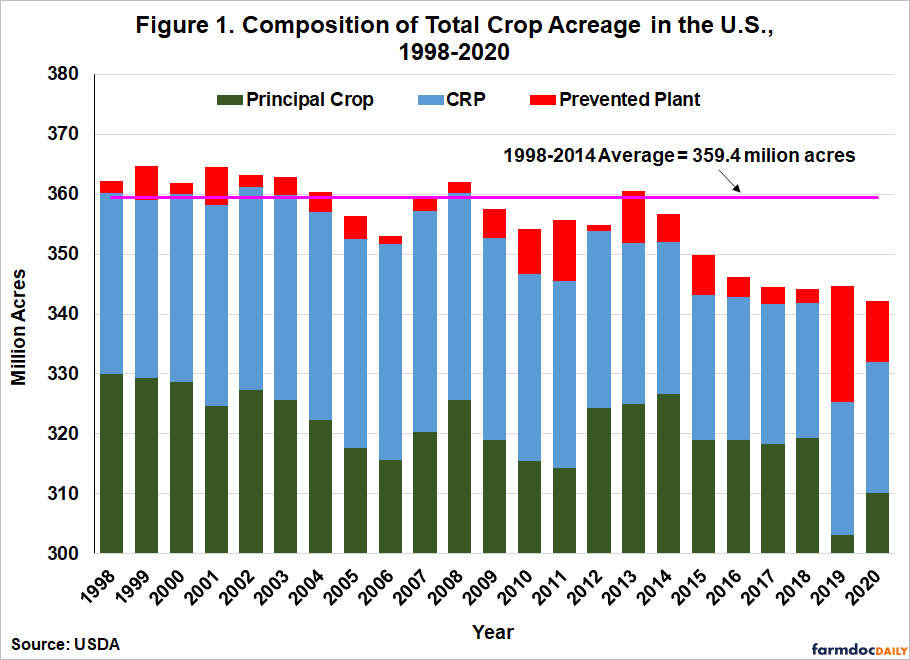
Prevented planting is a failure to plant an insured crop with the proper equipment by the final planting date designated in the insurance policy’s Special Provisions or during the late planting period, if applicable. https://farmdocdaily.illinois.edu/2021/06/estimating-total-crop-acres-in-the-us.html
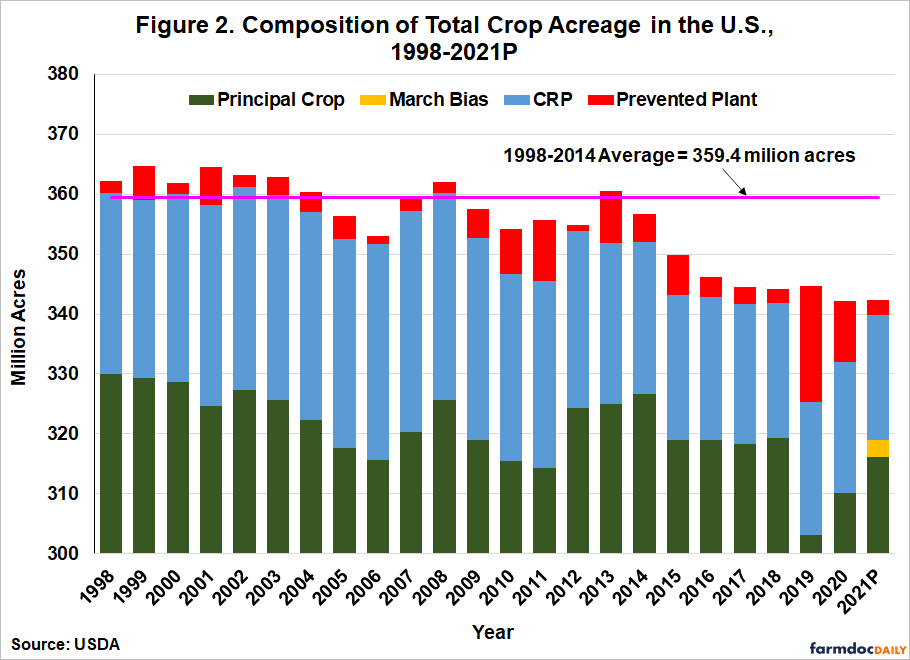 Native bees have specific preferences for plant species, for instance leafcutter bees pollinate alfalfa, canola, cranberries, onions, peas, blueberries and various other vegetables and melons. Bumble bees visit larkspur, iris, columbine, as well as tomatoes, peppers, blueberries, cranberries, broad beans and other “long throated” flowers. Several species of bumble bees are used in greenhouses, but they are expensive to order and maintain the many colonies that are needed inside these closed CEA’s (Controlled Environment Agriculture). Many native bees have long tongues and visit specific wildflowers.
Native bees have specific preferences for plant species, for instance leafcutter bees pollinate alfalfa, canola, cranberries, onions, peas, blueberries and various other vegetables and melons. Bumble bees visit larkspur, iris, columbine, as well as tomatoes, peppers, blueberries, cranberries, broad beans and other “long throated” flowers. Several species of bumble bees are used in greenhouses, but they are expensive to order and maintain the many colonies that are needed inside these closed CEA’s (Controlled Environment Agriculture). Many native bees have long tongues and visit specific wildflowers.
The squash bee is specifically designed to pollinate squash flowers but requires soft soil at the edge of the field in which to dig five to eight inch deep tunnels in which to deposit pollen and lay eggs. Although efficient at pollinating many cucurbit flowers, they only fly a quarter of a mile from their soil tunnels. If the edges of the field are treated with herbicide, plowed or mowed, the squash bees will not survive.
Mason bees are Spring time pollinators. They emerge from tubes or stems in early Spring and are mostly finished by early June. They only fly 100-300 yards from their nests. Leafcutter bees cut those curious perfectly round circles in redbud and rose leaves which they use to fold into cups to place eggs and pollen bundles into stems, holes in wood or the ground. They are active in mid-Summer (70°F) and pollinate flowers within 100 yards of their nest. Obviously, none of these bees can cover the tens of thousands of acres of orchards, fruit, vegetable and soybean fields in the United States alone.
Other pollinators such as syrphid flies, moths and beetles are far less efficient because their bodies are not hairy nor do the bodies have much contact with the pollen or stigmas. In fact, the USDA showed that the pollination services of non-Apis pollinators were valued at USD $3.44 billion, while honey bees contributed approximately $15 billion in the USA. Honey bees are responsible for pollinating over 80% of all flowering plants and 130 types of fruits and vegetables in the U.S. alone.
 Perhaps the most obvious point to the argument is that honey bees are by far the most efficient pollinator that can withstand regular management and long-distance trucking. What other insect can you pick up its nest, put it on a semi truck, drive it bouncing along for hundreds and hundreds of miles, unload it and have it still be alive, readjust to the new environment and go out and search for pollinator dependent flowers?
Perhaps the most obvious point to the argument is that honey bees are by far the most efficient pollinator that can withstand regular management and long-distance trucking. What other insect can you pick up its nest, put it on a semi truck, drive it bouncing along for hundreds and hundreds of miles, unload it and have it still be alive, readjust to the new environment and go out and search for pollinator dependent flowers?
Honey bees pollinate $20 billion worth of crops in the United States each year, including more than 130 types of fruits, nuts and vegetables. This of course, is in addition to the $3.2 million’s worth of honey produced in 2017 (USDA-National Agricultural Statistics Service (NASS)), beeswax, royal jelly, pollen and propolis that is harvested. Honey bees possess “flower fidelity” in that they will visit 50-100 apple blossoms, onion or carrot flowers or clover flowers in one trip instead of going to an apple flower then a clover then a mustard bloom, therefore the flowers visited by honey bees benefit by receiving only pollen from its cultivar or species, receiving maximum pollination. They can be placed in orchards or fields as needed to supply pollination for the entire field, then moved to meet the demands of another crop. Beehives are often important elements of urban gardens due to the pollination services they provide for backyard and community gardens, wildflowers and parks. Thanks to honey bees, birds, insects and many animals thrive on the fruits produced from the work of the honey bees.
Currently, 330 million people live in the United States with an estimated count of 400 million by 2050. Since 2000, the total area of farmland in U.S. has decreased annually. In that period, the total farmland area has decreased by almost 50 million acres, reaching a total of 893.4 million acres as of 2022. For the first time, the United States has imported more food than it has exported, meaning that we are depending upon other countries for food. In the monoculture system common in the U.S. and other advanced countries, honey bees are crucial to provide the pollination needed to feed animals and people. A study by Ritchie showed that crops are not pollinated sufficiently and that populations in many countries (including the U.S.), are undernourished due to insufficient pollination. Instead of arguing about honey bees or native bees, we should be concentrating on finding ways to keep farmland in production and provide more habitat for all pollinators.
References
https://ohioline.osu.edu/factsheet/ent-85
https://www.usda.gov/peoples-garden/pollinators/honey-bees#:~:text=Honeybees%20pollinate%20%2415%20billion%20worth,fruits%2C%20nuts%2C%20and%20vegetables
https://www.statista.com/statistics/196104/total-area-of-land-in-farms-in-the-us-since-2000/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8396518/pdf/insects-12-00688.pdf
https://www.usda.gov/sites/default/files/documents/Attractiveness-of-Agriculture-Crops-to-Pollinating-Bees-Report-FINAL-Web-Version-Jan-3-2018.pdf

Figure 1. Duan Copeland, Ph.D., inspecting hives in Tucson, AZ.
Researchers in Tucson, Arizona, are developing an AI-powered application to simplify and automate honey bee brood disease diagnosis and promote effective disease management. Leading the charge is Dr. Duan Copeland, a postdoctoral researcher in Dr. Kirk E. Anderson’s Lab, dedicated to diagnosing brood disease using only a smartphone photograph. “What can be challenging for even master beekeepers are the subtle visual cues produced by various disease-causing agents that target honey bee larvae, including bacteria, viruses and fungi” said Copeland. “We can train an AI program to recognize these differences in the same way an expert beekeeper or apiary inspector could.”
Connecting the dots
This brood disease research was sparked by Illinois State Apiary Inspector Jim Wellwood. In 2015, Jim came to visit the Tucson lab in conjunction with the annual apiary inspectors meeting. I was intrigued by our conversation, so I began a study of brood disease sampling throughout Illinois. Using these findings as preliminary data, the Anderson lab was awarded a large NIFA grant that included disease experts and co-PIs, Dr. Jay Evans and Dr. Meghan Milbrath, titled: Using big data to improve diagnosis of larval disease in honey bees. We began this project by high-throughput sequencing the bacterial microbiomes of third, fourth and fifth instar larvae to document disease progression across six diseased and one disease-free apiary. Simultaneously, we photographed the same larvae at high resolution (https://www.nature.com/articles/s41598-023-28085-2).
Of the apiaries we selected for deep sequencing, five of seven were experiencing EFB symptoms, one was asymptomatic and one had “melty” symptoms. Our approach sampling throughout larval development showed that EFB disease can manifest in a variety of ways. Similarly, recent results sequencing the genomes of EFB causative agent, M. plutonius, indicate that different bacterial strains have radically different personalities, and differ substantially in their ability to cause disease. The behavior of M. plutonius as a larval commensal, opportunist or pathogen is defined by a collection of virulence genes that allow it to exploit larvae. Additionally, there are genes in the M. plutonius genome that confer survival in the worker gut and hive environments, including royal jelly and honey.
Surprisingly, asymptomatic larval microbiomes frequently contained M. plutonius, including those sampled from asymptomatic apiaries and colonies. Some of this result came from the Tucson Lab Apiary where we rarely, if ever, experience EFB symptoms, yet a significant proportion (41%, 31 of 75) of the healthy larval microbiomes contained M. plutonius. Similarly, at one of the Illinois apiaries with no EFB symptoms, 75% (18 of 24) of asymptomatic larvae were positive for M. plutonius. The high-throughput method used to acquire this data is more sensitive than most tests and detects small amounts of bacteria providing a more complete picture of “who” is in there versus traditional culturing methods.
We discovered a variety of bacteria that occur as relatively harmless commensals in first through third instar larvae. As demonstrated for other species, these molecular patterns serve to “train” the immune system of developing larvae. We found that the progression of European Foulbrood (EFB) differed significantly by apiary due to secondary invaders and differences in beneficial bacteria. The discovered secondary invaders were very different from those identified with earlier culture-based methods with the single exception of Enterococcus faecalis, common to all EFB diseased apiaries. In fact, the presence and abundance of E. faecalis was positively associated with that of M. plutonius across multiple apiaries especially in asymptomatic larvae. This pattern of association is consistent with culture-based results and often indicates synergy among bacterial species. This pattern wasn’t limited to E. faecalis. A number of bacteria that commonly occur throughout the honey bee social network revealed their opportunistic nature, increasing with EFB disease progression. These species were Frischella perrara, the bacterium that commonly forms a scab in the worker gut where the host waste products are excreted, Apilactobacillus kunkeei an extremophilic bacterium that specializes on honey, and Fructobacillus fructosus, a lesser honey specialist that also occurs in healthy larvae but is scarce in the adult gut.
At one of the apiaries, one colony showed symptoms of Varroosis (Parasitic mite syndrome: PMS, also known as Idiopathic Brood Disease Syndrome: IBDS). Digging a little deeper, we found that Acute Bee Paralysis Virus (ABPV) levels were extraordinarily high in the symptomatic larvae, and correlated absolutely with the “melty, deflated and sunken” symptomology recorded by the apiary inspector. Critically, all of these comprehensively defined disease states and larval stages were recorded with high-resolution digital imaging. After many weeks examining these photos and disease states, we hypothesized that larval symptoms alone could be leveraged to accurately diagnose disease.
While it is known that paralytic viruses can infect larvae, honey bee science lacks an understanding of the microbes that either cause or result from “EFB-like” brood disease, molten brood, melty larvae, Parasitic mite syndrome and Varroosis. As part of our recent NIFA grant, we have been funded to unravel this can of worms; the crud, snot brood, melty brood, and mysteries that surround EFB-like brood disease. Our approach first relied on verbal descriptors, which we quickly determined are overly subjective, and woefully inadequate for diagnosis. We instead opted for the “picture does not lie” approach. In other words, a picture is comprised of pixels of quantifiable brightness, color and hue, arranged to form emergent properties that the computer program (or your mind) has been trained to interpret as shapes, in this case larvae, healthy or diseased.
Figure 2. Images of larvae and their associated bacterial microbiomes (vertical bars) from two different apiaries with brood disease: A) Larvae infected with EFB (black) and secondary invader Fructobacillus fructosus (dark green). B) Larvae infected with Acute Bee Paralysis Virus (ABPV) and associated opportunistic bacteria that carry antibiotic resistance genes including Serratia marcescens (red) and Frischella perrara (diagonal striped). The beekeeper treated apiary “B” with antibiotics, but the treatment was ineffective.
Traditional methods used to diagnose brood disease in the field require years of expertise. Like the rest of us novice beekeepers, perhaps it required a few seasons of dedicated beekeeping to form a reliable picture of the major honey bee brood diseases; conditions like chalkbrood, sacbrood, AFB, EFB, and EFB-like. Misdiagnosis is common, and prompts the unnecessary use of antibiotics, which disrupts the balance of the honey bee’s native microbiome. In turn, this disruption promotes the emergence of antibiotic-resistant strains of bacteria, further complicating disease management. In the case of the ABPV apiary (Fig. 2), the beekeeper assumed EFB disease and applied antibiotics. As illuminated by our metagenomic analysis, the antibiotics depleted the larval microbiome of beneficial bacteria and likely contributed to ABPV disease progression by removing the native barrier to opportunistic disease.
The future with AI
By leveraging the power of AI, the team aims to minimize the risk of misdiagnosis, reduce the reliance on antibiotics and ultimately contribute to more effective disease management practices for honey bees. Artificial Intelligence has already demonstrated success in honey bee research. Examples of image-based AI include identifying subspecies by wing patterns, detecting parasitic mites, tracking pollen foraging behavior and identification of comb resources.

Figure 3. Duan Copeland evaluating the efficiency of prototype AI models.
“Even a novice could look at diseased larvae and tell you some physical characteristics about it… this one looks yellow, this one is brown, this one’s melty,” he says. “An AI program can pick up on these same patterns, but it’s crucial we have the correct diagnosis and labeling to facilitate the AI training process.” Anderson’s team is collaborating with the Bee Disease Diagnostic Service in Beltsville, Maryland and apiary inspectors around the U.S. to expand their AI image training dataset by including a wider variety of AFB, EFB, viral and fungal disease. This partnership has broadened our collection of digital images and diverse brood disease phenotypes. In addition, diseased brood samples undergo molecular diagnostic screening and microbiome analysis in Anderson’s lab to ensure the correct diagnosis. By incorporating a more comprehensive range of disease symptoms, the researchers aim to continually update and improve the AI application’s diagnostic capabilities as a honey bee health management tool. “The honey bee pathosphere is constantly evolving, so we want to keep our AI database up to date with what is being seen out there,” Copeland says.
AI in the palm of your hands
The culmination of this research will be the development of a digital product, the Big Data Brood Disease (BDBD) app. This technology will assist the broader beekeeping community by substantially increasing the probability of an accurate diagnosis when the symptoms are unclear, or when a beekeeper has little experience and has not yet formed a reliable picture of various larval disease states. Following the recent big boom in hobbyist and beginner beekeepers across the nation, our tool will significantly reduce the development of antibiotic resistance in both pathogenic, opportunistic and beneficial bacteria. The early and rapid identification of disease outbreaks will facilitate the decision to apply antibiotics or alternative approved treatments. As a result, the BDBD app will contribute significantly to healthier bee populations and more sustainable beekeeping practices. If you are dealing with larval disease outbreaks, and would like to contribute to this project, please contact the Anderson lab NIFA project manager Brendon Mott: [email protected].
By: Thomas D. Seeley
For many years, I kept on the hillside above my little house outside Ithaca, New York, a special hive of bees. This hive was mounted on a platform scale of the sort that is normally used for weighing heavy packages. I visited this hive close to dark every evening from April to October, to weigh it and see how much food (nectar and pollen) the bees living inside this hive had collected that day. On many days, flowers yielding nectar and pollen were scarce, so this scale-hive colony gathered little food and I recorded in my notebook a weight gain of just a few ounces, or maybe even a weight loss. But on some days—such as during the explosion of dandelion flowers in May, the flowering of basswood trees in July and the profusion of goldenrod flowers in September—I recorded weight gains of several pounds. Most of these big weight gains marked strong collection of nectar. On one remarkable day in September 1992, for example, my scale-hive colony had amazing success… it took in more than 27 pounds of goldenrod nectar! I estimate that when ripened into honey, the bees’ gain from this one day’s labor was about 11 pounds of honey, which is enough to fill 15 plastic squeeze bears. Days like this tell us that a colony of honey bees must cope with booms and busts in its intake of nectar. This article describes a nifty communication behavior that helps a colony do so… the tremble dance.

Fig 1. A nectar storer (left) has inserted her tongue between the mouthparts of a nectar collector (right). The storer bee is imbibing the nectar that the collector bee is regurgitating from the full “honey stomach” in her swollen abdomen. Photo by Kenneth Lorenzen
The acquisition of nectar involves two distinct groups of bees: (1) the bees that work outside the hive collecting nectar and (2) the bees that work inside processing nectar (Fig. 1). The members of the first group, the nectar-forager bees, are among the oldest bees in a colony while the members of the second group, the nectar-storer bees, come from the ranks of a colony’s middle-aged bees. These two groups interact in the unloading area just inside the hive entrance. This is where the nectar foragers pass off their fresh nectar to the nectar storers (Fig. 2). These bees either distribute the nectar to hungry nestmates or they store it in the honey combs for future consumption.
Fig 2. The division of labor between nectar foragers and nectar stores in honey bee colonies.
The specialization by worker bees on different parts of the overall task of nectar acquisition—nectar collecting and nectar processing—boosts the efficiency of a colony’s honey making. It means, for example, that when a forager has located a patch of flowers laden with nectar, she can concentrate on exploiting these flowers before they fade, competitors arrive,or darkness falls, rather than spreading her efforts between collecting work and processing work.
At the same time, however, this division of labor creates a problem of work coordination within a colony. The rates of nectar collecting and nectar processing must be kept in balance for the overall operation of nectar acquisition to proceed smoothly. If the collecting rate exceeds the processing rate, then nectar foragers will experience delays in unloading upon return to the hive. Reciprocally, if the processing rate—or more precisely, the processing capacity—exceeds the collecting rate, then the nectar storers will experience delays in finding nectar foragers who need to be unloaded. Such problems of coordination associated with division of labor are not limited to honey bee colonies. They arise in human factories as well, since the efficiency of any multistage production process depends critically upon the absence of bottlenecks in the flow of items from one stage of the process to the next.
Some years ago, as part of my studies of the organization of nectar collection by honey bee colonies, I discovered that honey bees have a special communication signal that is produced by nectar foragers and is sent to potential nectar storers, and that helps a colony to keep its rates of nectar collecting and nectar processing well matched. This discovery was an especially sweet one because it also solved a long-standing mystery that had been raised in the 1920s by the Austrian zoologist and Nobel laureate, Karl von Frisch.
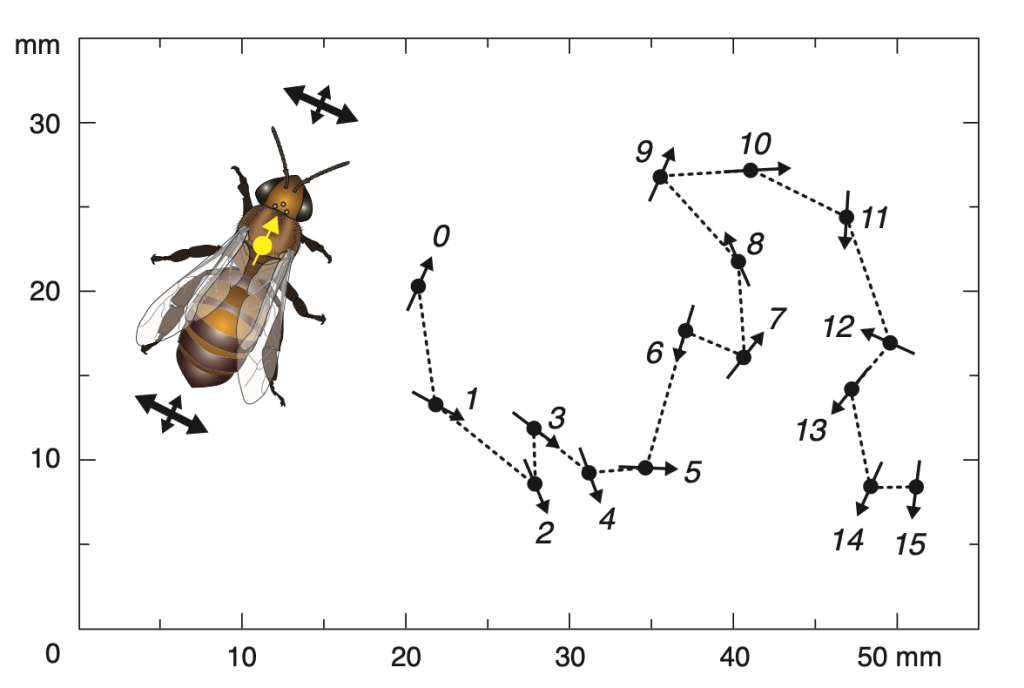
Fig 3. A 15-second record of a worker bee’s behavior while she performed the tremble dance. Drawing on left illustrates the striking, side-to-side trembles of her body. Track on right shows how this bee walked slowly across a comb and rotated her body by about 30 degrees every second or so. Scale: 50mm = 2 inches
In 1923, von Frisch published his first major report on the communication dances of honey bees. In this report, he described not only the famous waggle dance, by which foragers inform one another of the locations of rich food sources, but also a dance that he called the tremble dance (in German, der Zittertanz) (Fig. 3). My translation of his description of the tremble dance reads as follows:
At times one sees a strange behavior by bees who have returned home from a sugar water feeder or other goal. It is as if they had suddenly acquired the disease St. Vitus’s dance [chorea]. While they run about the combs in an irregular manner and with a slow tempo, their bodies, as a result of quivering movements of the legs, constantly make trembling movements forward and backward, and right and left. During this process they move about on four legs, with the forelegs, themselves trembling and shaking, held aloft approximately in the position in which a begging dog holds its forepaws. If they have brought home sugar water… often [they] will retain it until they have quieted down. The duration of this “tremble dance” is quite variable. I have seen instances where the phenomenon has died away after three to four minutes, then the bee appeared normal again and flew out of the hive. Usually, however, this dance lasts much longer and three times I have observed a bee tremble on the combs without interruption for three quarters of an hour.
The message of the tremble dance was a mystery to von Frisch, for although it seemed to be a communication signal, he was unable to identify its cause or detect any effect on other bees in the hive, despite having observed more than 60 bees perform this behavior. This led him to the tentative conclusion that the tremble dance gives the other bees no information. Some 40 years later, in his masterwork on the bees’ dances (von Frisch, 1967), he repeated this conclusion: “I think it [the tremble dance] tells the other bees nothing.” He noted that several other investigators had reported that tremble dances seemed to be the result of foragers experiencing adverse circumstances outside the hive—such as a marked deterioration of their food source—and he suggested that the tremble dance might be an incidental effect of a “nervous conflict” in these bees.
In 1987, I began to see that von Frisch’s conclusions about the tremble dance might be mistaken. I realized that this dance might play an important role in the organization of a colony’s foraging for nectar. My suspicions about this arose from some surprising results of an experiment that I performed with a colony living in an observation hive. When I removed most of the nectar storers from this colony and then observed the effects of this “surgery” on the colony’, I saw—as expected—that the nectar foragers had to search noticeably longer than usual to find nectar storers to get unloaded upon return to the hive. Their search times jumped from about 10 seconds to nearly 50 seconds. But, I also saw—to my surprise—that soon after the nectar foragers entered my observation hive and had difficulty finding nectar storers, they performed tremble dances. Wow! Furthermore, I saw that after about two hours, by which time the colony’s nectar foragers no longer needed to conduct lengthy searches to find nectar storers, the nectar foragers stopped performing tremble dances. It was clear that, somehow, the colony had replaced the nectar-storer bees that I had removed.
These serendipitous findings led me to form a two-part hypothesis regarding the tremble dance: first, its cause is nectar foragers experiencing long searches to find nectar storers; and second, its effect is to stimulate additional members of the colony to function as nectar storers. In other words, I proposed that the tremble dance serves to remove a bottleneck in the nectar acquisition process at the start of a honey flow by signaling a need for more workers to function as nectar-storer bees.
Four years later, in the Summer of 1991, I undertook an experiment designed to test my hypothesis. I moved a colony living in an observation hive to the Cranberry Lake Biological Station (CLBS), which is located in the Adirondack State Park, in northern New York. I took my study colony to this site because it is surrounded by nearly unbroken forests and lakes, so it is a place with very few flowers. Indeed, no honey bee colonies live around the CLBS, except those that I bring to it for my experiments. Here I can easily control the rate at which a colony’s nectar foragers bring nectar (sugar water) into their hive, because their only significant sources of food are the sugar-water feeders that I provide. And with the help of assistants tending the feeders, I can control the number of bees foraging at my feeders.
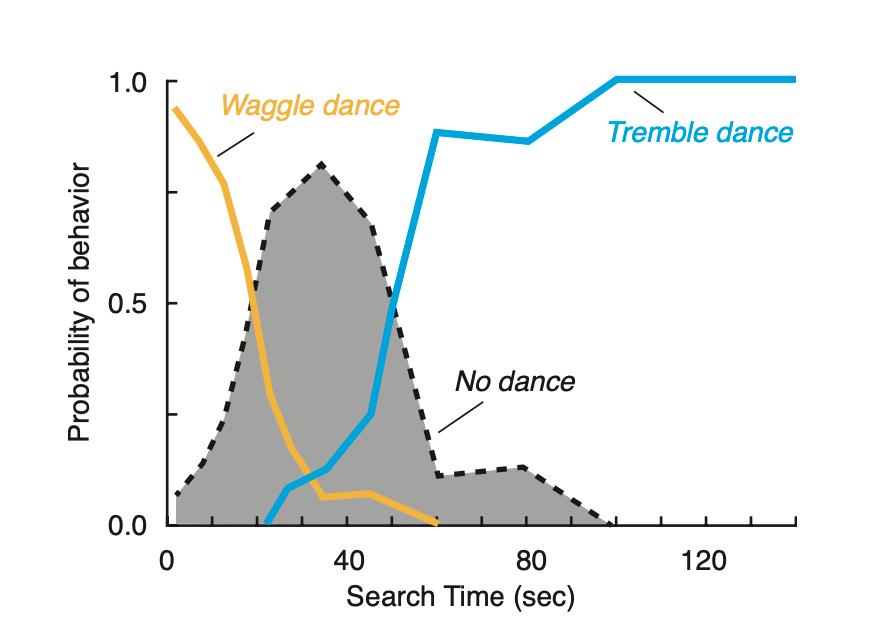
Fig 4. Dance behavior as a function of the in-hive search time for foragers that have visited a rich nectar source. Search times shorter than 20 seconds were followed by waggle dances, and search times longer than 50 seconds were followed by tremble dances.
To test my hypothesis about the cause of the tremble dance, I provided a colony living in an observation hive with two sugar-water feeders and I regulated the rate at which “nectar”-laden foragers returned to this hive by controlling the total number of bees visiting my two feeders. When this number was low (only 30 bees total), the forager bees quickly found nectar-storer bees (in just 10 seconds, on average) when they got home. And virtually all of the forager bees performed waggle dances. But when the number of bees visiting my two feeders was high (120 foragers total), these bees had difficulty finding nectar-storer bees (on average, each forager searched 45 seconds). And these forager bees no longer performed waggle dances; instead, they performed tremble dances! Overall, I saw that if a nectar forager located a nectar storer within 20 seconds of entering the hive, then she performed a waggle dance, but if she had to search for 40 seconds or more to find a nectar storer, then she performed a tremble dance (Fig. 4).
To test my hypothesis about the effect of the tremble dance, I again regulated the rate at which nectar foragers returned to the hive, but this time I counted the number of bees functioning as nectar storers on days with low and high levels of nectar-forager traffic, i.e., on days without and with tremble dancing. To count the nectar storers in my study colony, I replaced the glass on one side of my observation hive with a stiff nylon mesh (i.e., tulle, the material used to make tutus for ballet dancers) and then I daubed paint on the back of each bee that I saw unloading nectar from a forager. The effect of tremble dancing soon became clear. For example, on July 19, 1994 when the traffic level of nectar foragers was low (just three bees per minute entering the hive) and no tremble dances were performed, I labeled only 550 bees functioning as nectar storers. But on the next day, when the traffic level of nectar foragers was high (more than 25 bees per minute entering the hive) and more than 15 tremble dances were performed simultaneously within the hive, I labeled more than 1500 bees functioning as nectar storers.
These studies of the tremble dance deepened our appreciation of the complexity of the inner workings of a honey bee colony, especially of the bees’ social organization for making honey. We have long admired how foragers can share information about rich food sources by means of the waggle dance. We now understand that the effectiveness of the waggle dance in boosting a colony’s rate of nectar intake can create a problem for the bees, that of having too few nectar storers to handle the unloading needs of an enlarged group of nectar foragers. And we now understand that this problem is solved by means of a second dance produced by nectar foragers when they experience excessive delays in unloading—the tremble dance. Maybe there is even a lesson here for us. Perhaps our banks, grocery stores, and other places where customers must wait to be served should adopt a communication system like the bees’ tremble dance so that when long waiting lines develop the customers can call for additional tellers, cashiers, and the like, rather than just wait and quietly hope that more will soon appear.
Tom Seeley is a retired professor in the Department of Neurobiology and Behavior at Cornell University. This article is adapted from a chapter in his forthcoming book Piping Hot Bees and Boisterous Buzz Runners. 20 Mysteries of Honey Bee Behavior Solved. Princeton University Press.
]]>
